Disorganization of intercalated discs in dilated cardiomyopathy
- PMID: 34088908
- PMCID: PMC8178322
- DOI: 10.1038/s41598-021-90502-1
Disorganization of intercalated discs in dilated cardiomyopathy
Abstract
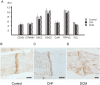
Dilated cardiomyopathy (DCM) is a primary myocardial disease, the pathology of which is left ventricular or biventricular dilation and impaired myocardial contractility. The clinical and pathological diagnosis of DCM is difficult, and other cardiac diseases must be ruled out. Several studies have reported pathological findings that are characteristic of DCM, including cardiomyocyte atrophy, nuclear pleomorphism, and interstitial fibrosis, but none of these findings are DCM-specific. In this study, we examined the morphological differences in the intercalated discs (ICDs) between three groups of patients, a DCM group, a chronic heart failure group, and a control group. A total of 22 autopsy cases, including five DCM cases, nine CHF cases and eight control cases, were retrieved from the archives of the Department of Pathology at Akita University, Japan. The morphological differences were examined using multiple methods: macroscopic examination, light microscopy, immunohistochemistry, electron microscopy, and gene expression analyses. We observed disorganized ICDs, clearly illustrated by N-cadherin immunostaining in the DCM group. "Reduction of N-cadherin immunostaining intensity" and "ICD scattering" was DCM-specific. The results suggest that disorganized ICDs contribute to the development of DCM, and that N-cadherin immunostaining is useful for determining the presence of disorganized ICDs and for the pathological diagnosis of DCM.
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Neutrophil Extracellular Traps in Myocardial Tissue Drive Cardiac Dysfunction and Adverse Outcomes in Patients With Heart Failure With Dilated Cardiomyopathy.Circ Heart Fail. 2024 Jun;17(6):e011057. doi: 10.1161/CIRCHEARTFAILURE.123.011057. Epub 2024 Jun 7. Circ Heart Fail. 2024. PMID: 38847093
-
Pathophysiology and pathological remodelling associated with dilated cardiomyopathy in broiler chickens predisposed to heart pump failure.Avian Pathol. 2020 Oct;49(5):428-439. doi: 10.1080/03079457.2020.1757620. Epub 2020 May 22. Avian Pathol. 2020. PMID: 32301624
-
Abnormal circadian rhythms exacerbate dilated cardiomyopathy by reducing the ventricular mechanical strength.Cardiovasc Res. 2024 Dec 31;120(17):2261-2277. doi: 10.1093/cvr/cvae212. Cardiovasc Res. 2024. PMID: 39270732
-
[Contribution of echocardiography to the diagnosis of patients with chronic heart failure].Ital Heart J Suppl. 2000 Oct;1(10):1311-6. Ital Heart J Suppl. 2000. PMID: 11068713 Review. Italian.
-
New and emerging biomarkers in left ventricular systolic dysfunction--insight into dilated cardiomyopathy.J Cardiovasc Transl Res. 2013 Aug;6(4):516-27. doi: 10.1007/s12265-013-9462-3. Epub 2013 Apr 23. J Cardiovasc Transl Res. 2013. PMID: 23609585 Free PMC article. Review.
Cited by
-
Sex-specific response to A1BG loss results in female dilated cardiomyopathy.Biol Sex Differ. 2025 Apr 23;16(1):27. doi: 10.1186/s13293-025-00713-8. Biol Sex Differ. 2025. PMID: 40270023 Free PMC article.
-
Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage.Open Life Sci. 2021 Aug 27;16(1):856-866. doi: 10.1515/biol-2021-0081. eCollection 2021. Open Life Sci. 2021. PMID: 34522779 Free PMC article.
-
Progressive cardiomyopathy with intercalated disc disorganization in a rat model of Becker dystrophy.EMBO Rep. 2024 Nov;25(11):4898-4920. doi: 10.1038/s44319-024-00249-9. Epub 2024 Oct 2. EMBO Rep. 2024. PMID: 39358550 Free PMC article.
-
Association of the TTN, PDK4, and RNF207 mutations with dilated cardiomyopathy in Dobermanns from the United Kingdom.PLoS One. 2025 Mar 13;20(3):e0319932. doi: 10.1371/journal.pone.0319932. eCollection 2025. PLoS One. 2025. PMID: 40080479 Free PMC article.
-
Upregulation of utrophin improves the phenotype of Duchenne muscular dystrophy hiPSC-derived CMs.Mol Ther Nucleic Acids. 2024 Jun 11;35(3):102247. doi: 10.1016/j.omtn.2024.102247. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39035791 Free PMC article.
References
-
- Ross, J., Jr. Adaptations of the left ventricle to chronic volume overload. Circ. Res.35(suppl II), 64–70. 10.1016/0002-9149(75)90774-2 - PubMed
-
- Patten RD, Udelson JE, Konstam MA. Ventricular remodeling and its prevention in the treatment of heart failure. Curr. Opin. Cardiol. 1998;13:162–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

