Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering
- PMID: 34088909
- PMCID: PMC8178384
- DOI: 10.1038/s41598-021-90903-2
Seaweed cellulose scaffolds derived from green macroalgae for tissue engineering
Abstract
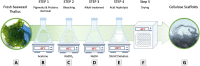
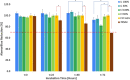
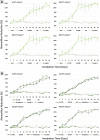
Extracellular matrix (ECM) provides structural support for cell growth, attachments and proliferation, which greatly impact cell fate. Marine macroalgae species Ulva sp. and Cladophora sp. were selected for their structural variations, porous and fibrous respectively, and evaluated as alternative ECM candidates. Decellularization-recellularization approach was used to fabricate seaweed cellulose-based scaffolds for in-vitro mammalian cell growth. Both scaffolds were confirmed nontoxic to fibroblasts, indicated by high viability for up to 40 days in culture. Each seaweed cellulose structure demonstrated distinct impact on cell behavior and proliferation rates. The Cladophora sp. scaffold promoted elongated cells spreading along its fibers' axis, and a gradual linear cell growth, while the Ulva sp. porous surface, facilitated rapid cell growth in all directions, reaching saturation at week 3. As such, seaweed-cellulose is an environmentally, biocompatible novel biomaterial, with structural variations that hold a great potential for diverse biomedical applications, while promoting aquaculture and ecological agenda.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ratner, B. D., Hoffman, A. S., Schoen, F. J. & Lemons, J. E. Biomaterials Science: An Introduction to Materials in Medicine (Elsevier, 2013).
-
- Lanza, R., Langer, R. & Vacanti, J. Principles of Tissue Engineering (Academic Press, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

