Inhibition of epithelial-mesenchymal transition in retinal pigment epithelial cells by a retinoic acid receptor-α agonist
- PMID: 34088917
- PMCID: PMC8178299
- DOI: 10.1038/s41598-021-90618-4
Inhibition of epithelial-mesenchymal transition in retinal pigment epithelial cells by a retinoic acid receptor-α agonist
Abstract
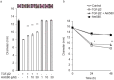
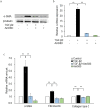
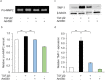
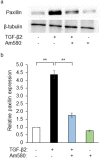
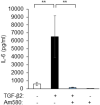
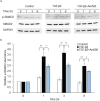
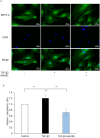
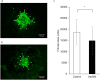
Epithelial-mesenchymal transition (EMT) in retinal pigment epithelial (RPE) cells plays a key role in proliferative retinal diseases such as age-related macular degeneration by contributing to subretinal fibrosis. To investigate the potential role of retinoic acid receptor-α (RAR-α) signaling in this process, we have now examined the effects of the RAR-α agonist Am580 on EMT induced by transforming growth factor-β2 (TGF-β2) in primary mouse RPE cells cultured in a three-dimensional type I collagen gel as well as on subretinal fibrosis in a mouse model. We found that Am580 inhibited TGF-β2-induced collagen gel contraction mediated by RPE cells. It also attenuated the TGF-β2-induced expression of the mesenchymal markers α-smooth muscle actin, fibronectin, and collagen type I; production of pro-matrix metalloproteinase 2 and interleukin-6; expression of the focal adhesion protein paxillin; and phosphorylation of SMAD2 in the cultured RPE cells. Finally, immunofluorescence analysis showed that Am580 suppressed both the TGF-β2-induced translocation of myocardin-related transcription factor-A (MRTF-A) from the cytoplasm to the nucleus of cultured RPE cells as well as subretinal fibrosis triggered by laser-induced photocoagulation in a mouse model. Our observations thus suggest that RAR-α signaling inhibits EMT in RPE cells and might attenuate the development of fibrosis associated with proliferative retinal diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist.J Mol Med (Berl). 2015 Jul;93(7):749-58. doi: 10.1007/s00109-015-1289-8. Epub 2015 May 8. J Mol Med (Berl). 2015. PMID: 25947075
-
Suppression of Epithelial-Mesenchymal Transition in Retinal Pigment Epithelial Cells by an MRTF-A Inhibitor.Invest Ophthalmol Vis Sci. 2019 Feb 1;60(2):528-537. doi: 10.1167/iovs.18-25678. Invest Ophthalmol Vis Sci. 2019. PMID: 30707754
-
Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells.J Neuroinflammation. 2022 Jul 14;19(1):182. doi: 10.1186/s12974-022-02546-3. J Neuroinflammation. 2022. PMID: 35831910 Free PMC article.
-
Yes-associated protein is essential for proliferative vitreoretinopathy development via the epithelial-mesenchymal transition in retinal pigment epithelial fibrosis.J Cell Mol Med. 2021 Nov;25(21):10213-10223. doi: 10.1111/jcmm.16958. Epub 2021 Oct 1. J Cell Mol Med. 2021. PMID: 34598306 Free PMC article.
-
Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review.
Cited by
-
JR5558 mice are a reliable model to investigate subretinal fibrosis.Sci Rep. 2024 Aug 13;14(1):18752. doi: 10.1038/s41598-024-66068-z. Sci Rep. 2024. PMID: 39138242 Free PMC article.
-
All-trans retinoic acid regulates the expression of MMP-2 and TGF-β2 via RDH5 in retinal pigment epithelium cells.Int J Ophthalmol. 2023 Jun 18;16(6):849-854. doi: 10.18240/ijo.2023.06.03. eCollection 2023. Int J Ophthalmol. 2023. PMID: 37332553 Free PMC article.
-
IKKβ Inhibition Attenuates Epithelial Mesenchymal Transition of Human Stem Cell-Derived Retinal Pigment Epithelium.Cells. 2023 Apr 13;12(8):1155. doi: 10.3390/cells12081155. Cells. 2023. PMID: 37190063 Free PMC article.
-
The role of the mTOR pathway in diabetic retinopathy.Front Med (Lausanne). 2022 Nov 1;9:973856. doi: 10.3389/fmed.2022.973856. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36388931 Free PMC article. Review.
-
Assessment of the level of apoptosis in differentiated pseudo-neuronal cells derived from neural stem cells under the influence of various inducers.Am J Stem Cells. 2024 Dec 15;13(6):250-270. doi: 10.62347/BPTG6174. eCollection 2024. Am J Stem Cells. 2024. PMID: 39850017 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

