Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit
- PMID: 34089064
- PMCID: PMC8178026
- DOI: 10.1007/s00134-021-06446-7
Moving from bytes to bedside: a systematic review on the use of artificial intelligence in the intensive care unit
Abstract
Purpose: Due to the increasing demand for intensive care unit (ICU) treatment, and to improve quality and efficiency of care, there is a need for adequate and efficient clinical decision-making. The advancement of artificial intelligence (AI) technologies has resulted in the development of prediction models, which might aid clinical decision-making. This systematic review seeks to give a contemporary overview of the current maturity of AI in the ICU, the research methods behind these studies, and the risk of bias in these studies.
Methods: A systematic search was conducted in Embase, Medline, Web of Science Core Collection and Cochrane Central Register of Controlled Trials databases to identify eligible studies. Studies using AI to analyze ICU data were considered eligible. Specifically, the study design, study aim, dataset size, level of validation, level of readiness, and the outcomes of clinical trials were extracted. Risk of bias in individual studies was evaluated by the Prediction model Risk Of Bias ASsessment Tool (PROBAST).
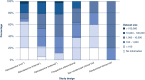
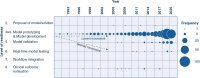
Results: Out of 6455 studies identified through literature search, 494 were included. The most common study design was retrospective [476 studies (96.4% of all studies)] followed by prospective observational [8 (1.6%)] and clinical [10 (2%)] trials. 378 (80.9%) retrospective studies were classified as high risk of bias. No studies were identified that reported on the outcome evaluation of an AI model integrated in routine clinical practice.
Conclusion: The vast majority of developed ICU-AI models remain within the testing and prototyping environment; only a handful were actually evaluated in clinical practice. A uniform and structured approach can support the development, safe delivery, and implementation of AI to determine clinical benefit in the ICU.
Keywords: Artificial intelligence; Clinical trials; Intensive care unit; Machine learning.
Conflict of interest statement
The authors declare that they have no conflicts of interest. DG has received speakers’ fees and travel expenses from Dräger, GE Healthcare (medical advisory board 2009–2012), Maquet, and Novalung (medical advisory board 2015–2018). All other authors declare no competing interests.
Figures


Comment in
-
Poor quality data, privacy, lack of certifications: the lethal triad of new technologies in intensive care.Intensive Care Med. 2021 Sep;47(9):1052-1053. doi: 10.1007/s00134-021-06473-4. Epub 2021 Jul 15. Intensive Care Med. 2021. PMID: 34264366 No abstract available.
References
-
- Adhikari NK, Rubenfeld GD. Worldwide demand for critical care. Curr Opin Crit Care. 2011;17:620–625. - PubMed
-
- Citerio G, Park S, Schmidt JM, Moberg R, Suarez JI, Le Roux PD. Data collection and interpretation. Neurocrit Care. 2015;22:360–368. - PubMed
-
- Fuhrmann V, Weber T, Roedl K, Motaabbed J, Tariparast A, Jarczak D, de Garibay APR, Kluwe J, Boenisch O, Herkner H, Kellum JA, Kluge S. Advanced organ support (ADVOS) in the critically ill: first clinical experience in patients with multiple organ failure. Ann Intensive Care. 2020;10:1. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

