Age and sex modify cellular proliferation responses to oxidative stress and glucocorticoid challenges in baboon cells
- PMID: 34089175
- PMCID: PMC8492861
- DOI: 10.1007/s11357-021-00395-1
Age and sex modify cellular proliferation responses to oxidative stress and glucocorticoid challenges in baboon cells
Abstract
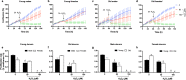
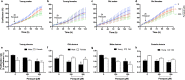
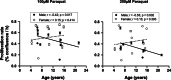
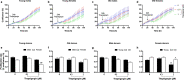
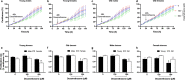
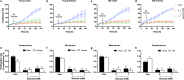
Aging is associated with progressive loss of cellular homeostasis resulting from intrinsic and extrinsic challenges. Lack of a carefully designed, well-characterized, precise, translational experimental model is a major limitation to understanding the cellular perturbations that characterize aging. Here, we tested the feasibility of primary fibroblasts isolated from nonhuman primates (baboons) as a model of cellular resilience in response to homeostatic challenge. Using a real-time live-cell imaging system, we precisely defined a protocol for testing effects of prooxidant compounds (e.g., hydrogen peroxide (H2O2), paraquat), thapsigargin, dexamethasone, and a low glucose environment on cell proliferation in fibroblasts derived from baboons across the life course (n = 11/sex). Linear regression analysis indicated that donor age significantly reduced the ability of cells to proliferate following exposure to H2O2 (50 and 100 µM) and paraquat (100 and 200 µM) challenges in cells from males (6.4-21.3 years; average lifespan 21 years) but not cells from females (4.3-15.9 years). Inhibitory effects of thapsigargin on cell proliferation were dependent on challenge duration (2 vs 24 h) and concentration (0.1 and 1 µM). Cells from older females (14.4-15.9 years) exhibited greater resilience to thapsigargin (1 µM; 24 h) and dexamethasone (500 µM) challenges than did those from younger females (4.3-6.7 years). The cell proliferation response to low glucose (1 mM) was reduced with age in both sexes. These data indicate that donor's chronological age and sex are important variables in determining fibroblast responses to metabolite and other challenges.
Keywords: Aging; Baboon; Cell proliferation; Fibroblast; Oxidative stress; Resilience.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Figures






Similar articles
-
Differential mitochondrial bioenergetics and cellular resilience in astrocytes, hepatocytes, and fibroblasts from aging baboons.bioRxiv [Preprint]. 2024 Feb 9:2024.02.06.579010. doi: 10.1101/2024.02.06.579010. bioRxiv. 2024. Update in: Geroscience. 2024 Oct;46(5):4443-4459. doi: 10.1007/s11357-024-01155-7. PMID: 38370705 Free PMC article. Updated. Preprint.
-
Cellular resilience and baboon aging.Aging (Albany NY). 2021 Nov 29;13(22):24482-24484. doi: 10.18632/aging.203728. Epub 2021 Nov 29. Aging (Albany NY). 2021. PMID: 34845113 Free PMC article. No abstract available.
-
Differential mitochondrial bioenergetics and cellular resilience in astrocytes, hepatocytes, and fibroblasts from aging baboons.Geroscience. 2024 Oct;46(5):4443-4459. doi: 10.1007/s11357-024-01155-7. Epub 2024 Apr 12. Geroscience. 2024. PMID: 38607532 Free PMC article.
-
The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress.IUBMB Life. 1999 Jul;48(1):41-7. doi: 10.1080/713803463. IUBMB Life. 1999. PMID: 10791914 Review.
-
Apoptosis in resistance arteries induced by hydrogen peroxide: greater resilience of endothelium versus smooth muscle.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1625-H1633. doi: 10.1152/ajpheart.00956.2020. Epub 2021 Feb 19. Am J Physiol Heart Circ Physiol. 2021. PMID: 33606587 Free PMC article. Review.
Cited by
-
Assessment of Oxidative Stress and Biometric Data in a Captive Colony of Hamadryas Baboons (Papio hamadryas Linnaeus, 1758) at the Ravenna Zoo Safari (Italy).Vet Sci. 2025 May 13;12(5):466. doi: 10.3390/vetsci12050466. Vet Sci. 2025. PMID: 40431559 Free PMC article.
-
Differential mitochondrial bioenergetics and cellular resilience in astrocytes, hepatocytes, and fibroblasts from aging baboons.bioRxiv [Preprint]. 2024 Feb 9:2024.02.06.579010. doi: 10.1101/2024.02.06.579010. bioRxiv. 2024. Update in: Geroscience. 2024 Oct;46(5):4443-4459. doi: 10.1007/s11357-024-01155-7. PMID: 38370705 Free PMC article. Updated. Preprint.
-
Developmental Programming-Aging Interactions Have Sex-Specific and Developmental Stage of Exposure Outcomes on Life Course Circulating Corticosterone and Dehydroepiandrosterone (DHEA) Concentrations in Rats Exposed to Maternal Protein-Restricted Diets.Nutrients. 2023 Mar 1;15(5):1239. doi: 10.3390/nu15051239. Nutrients. 2023. PMID: 36904238 Free PMC article.
-
Cellular resilience and baboon aging.Aging (Albany NY). 2021 Nov 29;13(22):24482-24484. doi: 10.18632/aging.203728. Epub 2021 Nov 29. Aging (Albany NY). 2021. PMID: 34845113 Free PMC article. No abstract available.
-
Differential mitochondrial bioenergetics and cellular resilience in astrocytes, hepatocytes, and fibroblasts from aging baboons.Geroscience. 2024 Oct;46(5):4443-4459. doi: 10.1007/s11357-024-01155-7. Epub 2024 Apr 12. Geroscience. 2024. PMID: 38607532 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

