Discovering fiber type architecture over the entire muscle using data-driven analysis
- PMID: 34089298
- PMCID: PMC9545503
- DOI: 10.1002/cyto.a.24465
Discovering fiber type architecture over the entire muscle using data-driven analysis
Abstract
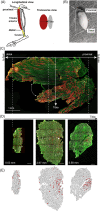
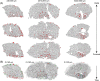
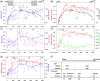
Skeletal muscle function is inferred from the spatial arrangement of muscle fiber architecture, which corresponds to myofiber molecular and metabolic features. Myofiber features are often determined using immunofluorescence on a local sampling, typically obtained from a median region. This median region is assumed to represent the entire muscle. However, it remains largely unknown to what extent this local sampling represents the entire muscle. We present a pipeline to study the architecture of muscle fiber features over the entire muscle, including sectioning, staining, imaging to image quantification and data-driven analysis with Myofiber type were identified by the expression of myosin heavy chain (MyHC) isoforms, representing contraction properties. We reconstructed muscle architecture from consecutive cross-sections stained for laminin and MyHC isoforms. Examining the entire muscle using consecutive cross-sections is extremely laborious, we provide consideration to reduce the dataset without loosing spatial information. Data-driven analysis with over 150,000 myofibers showed spatial variations in myofiber geometric features, myofiber type, and the distribution of neuromuscular junctions over the entire muscle. We present a workflow to study histological changes over the entire muscle using high-throughput imaging, image quantification, and data-driven analysis. Our results suggest that asymmetric spatial distribution of these features over the entire muscle could impact muscle function. Therefore, instead of a single sampling from a median region, representative regions covering the entire muscle should be investigated in future studies.
Keywords: data-driven analysis; muscle architecture; myofiber type; quantitative image analysis.
© 2021 The Authors. Cytometry Part A published by Wiley Periodicals LLC on behalf of International Society for Advancement of Cytometry.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


References
-
- Siebert T, Tomalka A, Stutzig N, Leichsenring K, Böl M. Changes in three‐dimensional muscle structure of rabbit gastrocnemius, flexor digitorum longus, and tibialis anterior during growth. J Mech Behav Biomed Mater. 2017;74:507–19. - PubMed
-
- Otten E. Concepts and models of functional architecture in skeletal muscle. Exerc Sport Sci Rev. 1988;16:89–137. - PubMed
-
- Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66. https://doi.org/10.1002/1097‐4598(200011)23:11<1647::aid‐mus1>3.0.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

