ARL15 modulates magnesium homeostasis through N-glycosylation of CNNMs
- PMID: 34089346
- PMCID: PMC8257531
- DOI: 10.1007/s00018-021-03832-8
ARL15 modulates magnesium homeostasis through N-glycosylation of CNNMs
Abstract
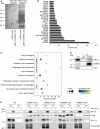
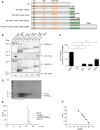
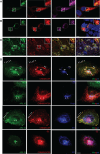
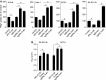
Cyclin M (CNNM1-4) proteins maintain cellular and body magnesium (Mg2+) homeostasis. Using various biochemical approaches, we have identified members of the CNNM family as direct interacting partners of ADP-ribosylation factor-like GTPase 15 (ARL15), a small GTP-binding protein. ARL15 interacts with CNNMs at their carboxyl-terminal conserved cystathionine-β-synthase (CBS) domains. In silico modeling of the interaction between CNNM2 and ARL15 supports that the small GTPase specifically binds the CBS1 and CNBH domains. Immunocytochemical experiments demonstrate that CNNM2 and ARL15 co-localize in the kidney, with both proteins showing subcellular localization in the endoplasmic reticulum, Golgi apparatus and the plasma membrane. Most importantly, we found that ARL15 is required for forming complex N-glycosylation of CNNMs. Overexpression of ARL15 promotes complex N-glycosylation of CNNM3. Mg2+ uptake experiments with a stable isotope demonstrate that there is a significant increase of 25Mg2+ uptake upon knockdown of ARL15 in multiple kidney cancer cell lines. Altogether, our results establish ARL15 as a novel negative regulator of Mg2+ transport by promoting the complex N-glycosylation of CNNMs.
Keywords: CNNM2; CNNM3; Glycosylation; Magnesium transport; Protein-protein interaction.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- PhD Scholarship/Fonds de Recherche du Québec - Santé
- #294027/Fonds de Recherche du Québec - Santé
- EJPRD2019-40/European Joint Program for Rare Disease
- NWO Veni 016.186.012. Vici 016.130.668/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NL)
- 2017.81.014/Dutch Diabetes Research Foundation
- PGC2018-096049-B-I00/Ministerio de Ciencia, Innovación y Universidades
- BIO-198, US-1254317 and US-1257019/Andalusian Government
- ERT-168503/CAPMC/ CIHR/Canada
- MOP-142497 and FDN-159923/CAPMC/ CIHR/Canada
- EJP RD COFUND-EJP N° 825575/European Union's Horizon 2020 research and innovation programme
- ERT-168503/CAPMC/ CIHR/Canada
- MOP-142497 and FDN-159923/CAPMC/ CIHR/Canada
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

