Feasibility of pharmacometabolomics to identify potential predictors of paclitaxel pharmacokinetic variability
- PMID: 34089352
- PMCID: PMC8319133
- DOI: 10.1007/s00280-021-04300-7
Feasibility of pharmacometabolomics to identify potential predictors of paclitaxel pharmacokinetic variability
Abstract
Purpose: Paclitaxel is a commonly used chemotherapy drug with substantial variability in pharmacokinetics (PK) that affects treatment efficacy and toxicity. Pharmacometabolomic signatures that explain PK variability could be used to individualize dosing to improve therapeutic outcomes. The objective of this study was to identify pretreatment metabolites or metabolomic signatures that explain variability in paclitaxel PK.
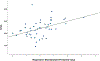
Methods: This analysis was conducted using data previously collected on a prospective observational study of 48 patients with breast cancer receiving weekly 80 mg/m2 paclitaxel infusions. Paclitaxel plasma concentrations were measured during the first infusion to estimate paclitaxel time above threshold (Tc>0.05) and maximum concentration (Cmax). Metabolites measured in pretreatment whole blood by nuclear magnetic resonance spectrometry were analyzed for an association with Tc>0.05 and Cmax using Pearson correlation followed by stepwise linear regression.
Results: Pretreatment creatinine, glucose, and lysine concentrations were positively correlated with Tc>0.05, while pretreatment betaine was negatively correlated and lactate was positively correlated with Cmax (all uncorrected p < 0.05). After stepwise elimination, creatinine was associated with Tc>0.05, while betaine and lactate were associated with Cmax (all p < 0.05).
Conclusion: This study identified pretreatment metabolites that may be associated with paclitaxel PK variability demonstrating feasibility of a pharmacometabolomics approach for understanding paclitaxel PK. However, identification of more robust pharmacometabolomic predictors will be required for broad and routine application for the clinical dosing of paclitaxel.
Keywords: Biomarkers; Breast cancer; Metabolomics; Nuclear magnetic resonance; Oncology; Personalized treatment.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
6. Conflicts of Interest
DLH has an unpaid, informal scientific collaboration with Saladaax Inc., a company that provides paclitaxel concentration measurement for therapeutic drug monitoring. Saladax had no role in this clinical study or secondary analysis.
Figures


References
-
- Albain K; Anderson S; Arriagada R; Barlow W; Bergh J; Bliss J; Buyse M; Cameron D; Carrasco E; Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 2012, 379, 432–444, doi: 10.1016/S0140-6736(11)61625-5. - DOI - PMC - PubMed
-
- Mielke S; Sparreboom A; Behringer D; Mross K Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Res 2005, 25, 4423–4427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

