Impact of Rotavirus Vaccine Introduction on Rotavirus Hospitalizations Among Children Under 5 Years of Age-World Health Organization African Region, 2008-2018
- PMID: 34089588
- PMCID: PMC11703079
- DOI: 10.1093/cid/ciab520
Impact of Rotavirus Vaccine Introduction on Rotavirus Hospitalizations Among Children Under 5 Years of Age-World Health Organization African Region, 2008-2018
Abstract
Background: Rotavirus is the leading cause of acute gastroenteritis (AGE) among children worldwide. Prior to rotavirus vaccine introduction, over one third of AGE hospitalizations in Africa were due to rotavirus. We describe the impact of rotavirus vaccines using data from the African Rotavirus Surveillance Network (ARSN).
Methods: For descriptive analysis, we included all sites reporting to ARSN for any length of time between 2008 and 2018. For vaccine impact analysis, continuous surveillance throughout the year was required to minimize potential bias due to enrollment of partial seasons and sites had to report a minimum of 100 AGE cases per year. We report the proportion of rotavirus AGE cases by year relative to vaccine introduction, and the relative reduction in the proportion of rotavirus AGE cases reported following vaccine introduction.
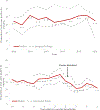
Results: From 2008 to 2018, 97 366 prospectively enrolled hospitalized children <5 years of age met the case definition for AGE, and 34.1% tested positive for rotavirus. Among countries that had introduced rotavirus vaccine, the proportion of hospitalized AGE cases positive for rotavirus declined from 39.2% in the prevaccine period to 25.3% in the postvaccine period, a 35.5% (95% confidence interval [CI]: 33.7-37.3) decline. No declines were observed among countries that had not introduced the vaccine over the 11-year period.
Conclusions: Rotavirus vaccine introduction led to large and consistent declines in the proportion of hospitalized AGE cases that are positive for rotavirus. To maximize the public health benefit of these vaccines, efforts to introduce rotavirus vaccines in the remaining countries in the region and to improve coverage should continue.
Keywords: Africa; acute gastroenteritis; hospitalization; rotavirus; vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Conflict of interest statement
Conflicts of interest: No authors report any conflicts of interest.
Figures
References
-
- Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. The New England journal of medicine 2010; 362:289–98. - PubMed
-
- World Health Organization. Rotavirus vaccines: an update. Weekly Epidemiological Record 2009; 84:533–7. - PubMed
-
- Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous