Naturally Acquired SARS-CoV-2 Immunity Persists for Up to 11 Months Following Infection
- PMID: 34089610
- PMCID: PMC8195007
- DOI: 10.1093/infdis/jiab295
Naturally Acquired SARS-CoV-2 Immunity Persists for Up to 11 Months Following Infection
Abstract
Background: Characterizing the kinetics of the antibody response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is of critical importance to developing strategies that may mitigate the public health burden of coronavirus disease 2019 (COVID-19). We conducted a prospective, longitudinal analysis of COVID-19 convalescent plasma donors at multiple time points over an 11-month period to determine how circulating antibody levels change over time following natural infection.
Methods: From April 2020 to February 2021, we enrolled 228 donors. At each study visit, subjects either donated plasma or had study samples drawn only. Anti-SARS-CoV-2 donor testing was performed using the VITROS Anti-SARS-CoV-2 Total and IgG assays and an in-house fluorescence reduction neutralization assay.
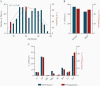
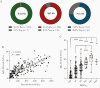
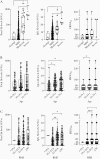
Results: Anti-SARS-CoV-2 antibodies were identified in 97% of COVID-19 convalescent donors at initial presentation. In follow-up analyses, of 116 donors presenting at repeat time points, 91.4% had detectable IgG levels up to 11 months after symptom recovery, while 63% had detectable neutralizing titers; however, 25% of donors had neutralizing levels that dropped to an undetectable titer over time.
Conclusions: Our data suggest that immunological memory is acquired in most individuals infected with SARS-CoV-2 and is sustained in a majority of patients for up to 11 months after recovery. Clinical Trials Registration. NCT04360278.
Keywords: COVID-19; convalescent plasma; immunological memory; neutralizing; serology.
Published by Oxford University Press for the Infectious Diseases Society of America 2021.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

