The neuroscience of social feelings: mechanisms of adaptive social functioning
- PMID: 34089764
- PMCID: PMC8388127
- DOI: 10.1016/j.neubiorev.2021.05.028
The neuroscience of social feelings: mechanisms of adaptive social functioning
Abstract
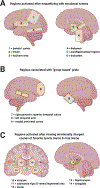
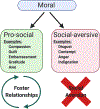
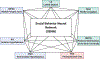
Social feelings have conceptual and empirical connections with affect and emotion. In this review, we discuss how they relate to cognition, emotion, behavior and well-being. We examine the functional neuroanatomy and neurobiology of social feelings and their role in adaptive social functioning. Existing neuroscience literature is reviewed to identify concepts, methods and challenges that might be addressed by social feelings research. Specific topic areas highlight the influence and modulation of social feelings on interpersonal affiliation, parent-child attachments, moral sentiments, interpersonal stressors, and emotional communication. Brain regions involved in social feelings were confirmed by meta-analysis using the Neurosynth platform for large-scale, automated synthesis of functional magnetic resonance imaging data. Words that relate specifically to social feelings were identfied as potential research variables. Topical inquiries into social media behaviors, loneliness, trauma, and social sensitivity, especially with recent physical distancing for guarding public and personal health, underscored the increasing importance of social feelings for affective and second person neuroscience research with implications for brain development, physical and mental health, and lifelong adaptive functioning.
Keywords: Emotional communication; Empathy; Interpersonal stressors; Loneliness; Moral sentiments; Parent-child attachment; Second person neuroscience; Social affiliation; Social feelings; Social influence; Social media; Trauma.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


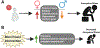
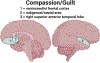

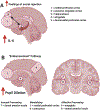


References
-
- Adkins-Regan E, 2009. Neuroendocrinology of social behavior. ILAR J. 50, 5–14. - PubMed
-
- Ainsworth MD, Bell SM, 1970. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 41, 49–67. - PubMed
-
- Albers HE, 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav 61, 283–292. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

