High intensity muscle stimulation activates a systemic Nrf2-mediated redox stress response
- PMID: 34089788
- PMCID: PMC8355059
- DOI: 10.1016/j.freeradbiomed.2021.05.039
High intensity muscle stimulation activates a systemic Nrf2-mediated redox stress response
Abstract
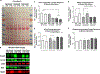
High intensity exercise is a popular mode of exercise to elicit similar or greater adaptive responses compared to traditional moderate intensity continuous exercise. However, the molecular mechanisms underlying these adaptive responses are still unclear. The purpose of this pilot study was to compare high and low intensity contractile stimulus on the Nrf2-mediated redox stress response in mouse skeletal muscle. An intra-animal design was used to control for variations in individual responses to muscle stimulation by comparing a stimulated limb (STIM) to the contralateral unstimulated control limb (CON). High Intensity (HI - 100Hz), Low Intensity (LI - 50Hz), and Naïve Control (NC - Mock stimulation vs CON) groups were used to compare these effects on Nrf2-ARE binding, Keap1 protein, and downstream gene and protein expression of Nrf2 target genes. Muscle stimulation significantly increased Nrf2-ARE binding in LI-STIM compared to LI-CON (p = 0.0098), while Nrf2-ARE binding was elevated in both HI-CON and HI-STIM compared to NC (p = 0.0007). The Nrf2-ARE results were mirrored in the downregulation of Keap1, where Keap1 expression in HI-CON and HI-STIM were both significantly lower than NC (p = 0.008) and decreased in LI-STIM compared to LI-CON (p = 0.015). In addition, stimulation increased NQO1 protein compared to contralateral control regardless of stimulation intensity (p = 0.019), and HO1 protein was significantly higher in high intensity compared to the Naïve control group (p = 0.002). Taken together, these data suggest a systemic redox signaling exerkine is activating Nrf2-ARE binding and is intensity gated, where Nrf2-ARE activation in contralateral control limbs were only seen in the HI group. Other research in exercise induced Nrf2 signaling support the general finding that Nrf2 is activated in peripheral tissues in response to exercise, however the specific exerkine responsible for the systemic signaling effects is not known. Future work should aim to delineate these redox sensitive systemic signaling mechanisms.
Keywords: High intensity exercise; Muscle contraction; Nrf2-Keap1; Redox signaling.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors report no conflict of interest.
Figures

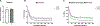

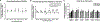
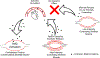
Similar articles
-
A Quantitative Proteomics Approach to Gain Insight into NRF2-KEAP1 Skeletal Muscle System and Its Cysteine Redox Regulation.Genes (Basel). 2021 Oct 21;12(11):1655. doi: 10.3390/genes12111655. Genes (Basel). 2021. PMID: 34828261 Free PMC article.
-
Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle.J Physiol. 2020 Dec;598(23):5427-5451. doi: 10.1113/JP280176. Epub 2020 Sep 23. J Physiol. 2020. PMID: 32893883 Free PMC article.
-
p62/SQSTM1 and Nrf2 are essential for exercise-mediated enhancement of antioxidant protein expression in oxidative muscle.FASEB J. 2019 Jul;33(7):8022-8032. doi: 10.1096/fj.201900133R. Epub 2019 Mar 26. FASEB J. 2019. PMID: 30913396
-
Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy.Biomed Pharmacother. 2020 Mar;123:109732. doi: 10.1016/j.biopha.2019.109732. Epub 2020 Jan 13. Biomed Pharmacother. 2020. PMID: 31945695 Review.
-
Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression.Am J Nephrol. 2017;45(6):473-483. doi: 10.1159/000475890. Epub 2017 May 13. Am J Nephrol. 2017. PMID: 28502971 Review.
Cited by
-
Antioxidant-Rich Functional Foods and Exercise: Unlocking Metabolic Health Through Nrf2 and Related Pathways.Int J Mol Sci. 2025 Jan 27;26(3):1098. doi: 10.3390/ijms26031098. Int J Mol Sci. 2025. PMID: 39940866 Free PMC article. Review.
-
Exercise and vascular function in sedentary lifestyles in humans.Pflugers Arch. 2023 Jul;475(7):845-856. doi: 10.1007/s00424-023-02828-6. Epub 2023 Jun 5. Pflugers Arch. 2023. PMID: 37272982 Review.
-
Phytochemicals and modulation of exercise-induced oxidative stress: a novel overview of antioxidants.Am J Transl Res. 2022 Nov 15;14(11):8292-8314. eCollection 2022. Am J Transl Res. 2022. PMID: 36505319 Free PMC article. Review.
-
The Role of Nrf2 in Skeletal Muscle on Exercise Capacity.Antioxidants (Basel). 2021 Oct 27;10(11):1712. doi: 10.3390/antiox10111712. Antioxidants (Basel). 2021. PMID: 34829582 Free PMC article.
-
Effects of exercise on body fat percentage and cardiorespiratory fitness in sedentary adults: a systematic review and network meta-analysis.Front Public Health. 2025 Jul 17;13:1624562. doi: 10.3389/fpubh.2025.1624562. eCollection 2025. Front Public Health. 2025. PMID: 40746688 Free PMC article.
References
-
- Merry TL, MacRae C, Pham T, Hedges CP, Ristow M, Deficiency in ROS-sensing nuclear factor erythroid 2-like 2 causes altered glucose and lipid homeostasis following exercise training, American Journal of Physiology-Cell Physiology 318(2) (2019) C337–C345. - PubMed
-
- Nikolaidis MG, Margaritelis NV, Matsakas A, Quantitative Redox Biology of Exercise, International journal of sports medicine (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

