Cerebrospinal fluid in COVID-19 neurological complications: Neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm
- PMID: 34090021
- PMCID: PMC8166041
- DOI: 10.1016/j.jns.2021.117517
Cerebrospinal fluid in COVID-19 neurological complications: Neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm
Abstract
Objective: To study in cerebrospinal fluid (CSF) of COVID-19 subjects if a "cytokine storm" or neuroinflammation are implicated in pathogenesis of neurological complications.
Methods: Cross-sectional study of CSF neuroinflammatory profiles from 18 COVID-19 subjects with neurological complications categorized by diagnosis (stroke, encephalopathy, headache) and illness severity. COVID-19 CSF was compared with CSF from healthy, infectious and neuroinflammatory disorders and stroke controls (n = 82). Cytokines (IL-6, TNFα, IFNγ, IL-10, IL-12p70, IL-17A), inflammation and coagulation markers (high-sensitivity-C Reactive Protein [hsCRP], ferritin, fibrinogen, D-dimer, Factor VIII) and neurofilament light chain (NF-L), were quantified. SARS-CoV2 RNA and SARS-CoV2 IgG and IgA antibodies in CSF were tested with RT-PCR and ELISA.
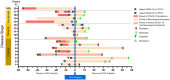
Results: CSF from COVID-19 subjects showed absence of pleocytosis or specific increases in pro-inflammatory markers (IL-6, ferritin, or D-dimer). Although pro-inflammatory cytokines (IL-6, TNFα, IL-12p70) and IL-10 were increased in CSF of stroke COVID-19 subjects, a similar increase was observed in non-COVID-19 stroke subjects. Anti-SARS-CoV2 antibodies in CSF of COVID-19 subjects (77%) were observed despite no evidence of SARS-CoV2 viral RNA. CSF-NF-L was elevated in subjects with stroke and critical COVID-19 as compared to controls and other COVID-19 severity categories. CSF-hsCRP was present in all subjects with critical stages of COVID-19 (7/18) but only in 1/82 controls.
Conclusion: The paucity of neuroinflammatory changes in CSF of COVID-19 subjects and lack of SARS-CoV2 RNA do not support the presumed neurovirulence of SARS-CoV2 or neuroinflammation in pathogenesis of neurological complications in COVID-19. The role of CSF SARS-CoV2 IgG antibodies and mechanisms of neuronal damage are still undetermined.
Keywords: COVID-19; Cerebrospinal fluid; Cytokine storm; Cytokines; Neuroinflammation.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures


Update of
-
Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation.medRxiv [Preprint]. 2021 Jan 12:2021.01.10.20249014. doi: 10.1101/2021.01.10.20249014. medRxiv. 2021. Update in: J Neurol Sci. 2021 Aug 15;427:117517. doi: 10.1016/j.jns.2021.117517. PMID: 33469596 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

