Activity of Plasmodium vivax promoter elements in Plasmodium knowlesi, and a centromere-containing plasmid that expresses NanoLuc throughout the parasite life cycle
- PMID: 34090438
- PMCID: PMC8180018
- DOI: 10.1186/s12936-021-03773-4
Activity of Plasmodium vivax promoter elements in Plasmodium knowlesi, and a centromere-containing plasmid that expresses NanoLuc throughout the parasite life cycle
Abstract
Background: Plasmodium knowlesi is now the major cause of human malaria in Malaysia, complicating malaria control efforts that must attend to the elimination of multiple Plasmodium species. Recent advances in the cultivation of P. knowlesi erythrocytic-stage parasites in vitro, transformation with exogenous DNA, and infection of mosquitoes with gametocytes from culture have opened up studies of this pathogen without the need for resource-intensive and costly non-human primate (NHP) models. For further understanding and development of methods for parasite transformation in malaria research, this study examined the activity of various trans-species transcriptional control sequences and the influence of Plasmodium vivax centromeric (pvcen) repeats in plasmid-transfected P. knowlesi parasites.
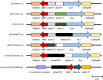
Methods: In vitro cultivated P. knowlesi parasites were transfected with plasmid constructs that incorporated Plasmodium vivax or Plasmodium falciparum 5' UTRs driving the expression of bioluminescence markers (firefly luciferase or Nanoluc). Promoter activities were assessed by bioluminescence, and parasites transformed with human resistant allele dihydrofolate reductase-expressing plasmids were selected using antifolates. The stability of transformants carrying pvcen-stabilized episomes was assessed by bioluminescence over a complete parasite life cycle through a rhesus macaque monkey, mosquitoes, and a second rhesus monkey.
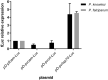
Results: Luciferase expression assessments show that certain P. vivax promoter regions, not functional in the more evolutionarily-distant P. falciparum, can drive transgene expression in P. knowlesi. Further, pvcen repeats may improve the stability of episomal plasmids in P. knowlesi and support detection of NanoLuc-expressing elements over the full parasite life cycle from rhesus macaque monkeys to Anopheles dirus mosquitoes and back again to monkeys. In assays of drug responses to chloroquine, G418 and WR9910, anti-malarial half-inhibitory concentration (IC50) values of blood stages measured by NanoLuc activity proved comparable to IC50 values measured by the standard SYBR Green method.
Conclusion: All three P. vivax promoters tested in this study functioned in P. knowlesi, whereas two of the three were inactive in P. falciparum. NanoLuc-expressing, centromere-stabilized plasmids may support high-throughput screenings of P. knowlesi for new anti-malarial agents, including compounds that can block the development of mosquito- and/or liver-stage parasites.
Keywords: Antimalarial drug response assays; Genetic transformation; Heterologous transfection; In vitro growth assays; Luciferase expression; Transgenic parasites.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Genetic Manipulation of Non-Falciparum Human Malaria Parasites.Front Cell Infect Microbiol. 2021 Aug 30;11:680460. doi: 10.3389/fcimb.2021.680460. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34527600 Free PMC article. Review.
-
Validation of Plasmodium vivax centromere and promoter activities using Plasmodium yoelii.PLoS One. 2019 Dec 20;14(12):e0226884. doi: 10.1371/journal.pone.0226884. eCollection 2019. PLoS One. 2019. PMID: 31860644 Free PMC article.
-
Infection of mosquitoes from in vitro cultivated Plasmodium knowlesi H strain.Int J Parasitol. 2018 Jul;48(8):601-610. doi: 10.1016/j.ijpara.2018.02.004. Epub 2018 Apr 30. Int J Parasitol. 2018. PMID: 29723510 Free PMC article.
-
Transfection of the primate malaria parasite Plasmodium knowlesi using entirely heterologous constructs.J Exp Med. 1997 Apr 21;185(8):1499-503. doi: 10.1084/jem.185.8.1499. J Exp Med. 1997. PMID: 9126931 Free PMC article.
-
Functional genomics of simian malaria parasites and host-parasite interactions.Brief Funct Genomics. 2019 Sep 24;18(5):270-280. doi: 10.1093/bfgp/elz013. Brief Funct Genomics. 2019. PMID: 31241151 Free PMC article. Review.
Cited by
-
Genetic Manipulation of Non-Falciparum Human Malaria Parasites.Front Cell Infect Microbiol. 2021 Aug 30;11:680460. doi: 10.3389/fcimb.2021.680460. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34527600 Free PMC article. Review.
-
Transfection Models to Investigate Plasmodium vivax-Type Dormant Liver Stage Parasites.Pathogens. 2023 Aug 22;12(9):1070. doi: 10.3390/pathogens12091070. Pathogens. 2023. PMID: 37764878 Free PMC article. Review.
-
Synthesis, Structure−Activity Relationships, and Parasitological Profiling of Brussonol Derivatives as New Plasmodium falciparum Inhibitors.Pharmaceuticals (Basel). 2022 Jun 30;15(7):814. doi: 10.3390/ph15070814. Pharmaceuticals (Basel). 2022. PMID: 35890113 Free PMC article.
References
-
- WHO. World malaria report 2017. Geneva: World Health Organization; 2017: http://apps.who.int/iris/bitstream/10665/259492/1/9789241565523-eng.pdf?....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

