Translational precision medicine: an industry perspective
- PMID: 34090480
- PMCID: PMC8179706
- DOI: 10.1186/s12967-021-02910-6
Translational precision medicine: an industry perspective
Abstract
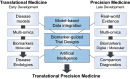
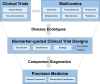
In the era of precision medicine, digital technologies and artificial intelligence, drug discovery and development face unprecedented opportunities for product and business model innovation, fundamentally changing the traditional approach of how drugs are discovered, developed and marketed. Critical to this transformation is the adoption of new technologies in the drug development process, catalyzing the transition from serendipity-driven to data-driven medicine. This paradigm shift comes with a need for both translation and precision, leading to a modern Translational Precision Medicine approach to drug discovery and development. Key components of Translational Precision Medicine are multi-omics profiling, digital biomarkers, model-based data integration, artificial intelligence, biomarker-guided trial designs and patient-centric companion diagnostics. In this review, we summarize and critically discuss the potential and challenges of Translational Precision Medicine from a cross-industry perspective.
Keywords: Artificial intelligence; Biomarkers; Companion diagnostics; Digital biomarkers; Drug development; Modeling; Multi-omics; Pharmaceutical industry; Precision medicine; Translational medicine.
Conflict of interest statement
The authors declare that there are no competing interests. DH, VdL, AK, JL, SC, EF, RS, MF and MH work for Novartis, LP and AR work for Roche.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

