"Don't Phos Over Tau": recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer's disease and other tauopathies
- PMID: 34090488
- PMCID: PMC8180161
- DOI: 10.1186/s13024-021-00460-5
"Don't Phos Over Tau": recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer's disease and other tauopathies
Abstract
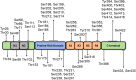
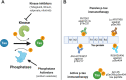
Phosphorylation is one of the most prevalent post-translational modifications found in aggregated tau isolated from Alzheimer's disease (AD) patient brains. In tauopathies like AD, increased phosphorylation or hyperphosphorylation can contribute to microtubule dysfunction and is associated with tau aggregation. In this review, we provide an overview of the structure and functions of tau protein as well as the physiologic roles of tau phosphorylation. We also extensively survey tau phosphorylation sites identified in brain tissue and cerebrospinal fluid from AD patients compared to age-matched healthy controls, which may serve as disease-specific biomarkers. Recently, new assays have been developed to measure minute amounts of specific forms of phosphorylated tau in both cerebrospinal fluid and plasma, which could potentially be useful for aiding clinical diagnosis and monitoring disease progression. Additionally, multiple therapies targeting phosphorylated tau are in various stages of clinical trials including kinase inhibitors, phosphatase activators, and tau immunotherapy. With promising early results, therapies that target phosphorylated tau could be useful at slowing tau hyperphosphorylation and aggregation in AD and other tauopathies.
Keywords: Alzheimer’s disease; Cerebrospinal fluid; Frontotemporal lobar degeneration; Kinase inhibitor; Phosphatase activator; Plasma; Tau immunotherapy; Tau phosphorylation; Tauopathy.
Conflict of interest statement
We declare no conflict of interest in this manuscript.
Figures

Similar articles
-
Post-translational modifications of tau protein in Alzheimer's disease.J Neural Transm (Vienna). 2005 Jun;112(6):813-38. doi: 10.1007/s00702-004-0221-0. Epub 2004 Oct 27. J Neural Transm (Vienna). 2005. PMID: 15517432 Review.
-
A new non-aggregative splicing isoform of human Tau is decreased in Alzheimer's disease.Acta Neuropathol. 2021 Jul;142(1):159-177. doi: 10.1007/s00401-021-02317-z. Epub 2021 May 2. Acta Neuropathol. 2021. PMID: 33934221 Free PMC article.
-
PTI-125 Reduces Biomarkers of Alzheimer's Disease in Patients.J Prev Alzheimers Dis. 2020;7(4):256-264. doi: 10.14283/jpad.2020.6. J Prev Alzheimers Dis. 2020. PMID: 32920628 Clinical Trial.
-
Novel aspects of the phosphorylation and structure of pathological tau: implications for tauopathy biomarkers.FEBS Open Bio. 2024 Feb;14(2):181-193. doi: 10.1002/2211-5463.13667. Epub 2023 Jul 8. FEBS Open Bio. 2024. PMID: 37391389 Free PMC article. Review.
-
A current view on Tau protein phosphorylation in Alzheimer's disease.Curr Opin Neurobiol. 2021 Aug;69:131-138. doi: 10.1016/j.conb.2021.03.003. Epub 2021 Apr 21. Curr Opin Neurobiol. 2021. PMID: 33892381 Review.
Cited by
-
Protein Misfolding in Pregnancy: Current Insights, Potential Mechanisms, and Implications for the Pathogenesis of Preeclampsia.Molecules. 2024 Jan 26;29(3):610. doi: 10.3390/molecules29030610. Molecules. 2024. PMID: 38338354 Free PMC article. Review.
-
Tau-targeting nanoparticles for treatment of Alzheimer's disease.Exploration (Beijing). 2024 Jun 21;5(2):20230137. doi: 10.1002/EXP.20230137. eCollection 2025 Apr. Exploration (Beijing). 2024. PMID: 40395755 Free PMC article. Review.
-
Development and characterization of novel anti-acetylated tau monoclonal antibodies to probe pathogenic tau species in Alzheimer's disease.Acta Neuropathol Commun. 2024 Oct 12;12(1):163. doi: 10.1186/s40478-024-01865-1. Acta Neuropathol Commun. 2024. PMID: 39396065 Free PMC article.
-
Recent Advances in Mass Spectrometry-Based Studies of Post-Translational Modifications in Alzheimer's Disease.Mol Cell Proteomics. 2025 Jul;24(7):101003. doi: 10.1016/j.mcpro.2025.101003. Epub 2025 May 29. Mol Cell Proteomics. 2025. PMID: 40449795 Free PMC article. Review.
-
Drosophila appear resistant to trans-synaptic tau propagation.Brain Commun. 2024 Aug 8;6(4):fcae256. doi: 10.1093/braincomms/fcae256. eCollection 2024. Brain Commun. 2024. PMID: 39130515 Free PMC article.
References
-
- Hanger DP, Betts JC, Loviny TLF, Blackstock WP, Anderton BH. New phosphorylation sites identified in Hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using Nanoelectrospray mass spectrometry. J Neurochem. 2002;71(6):2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

