Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress
- PMID: 34090506
- PMCID: PMC8180036
- DOI: 10.1186/s13045-021-01097-z
Antibody-drug conjugates for the treatment of lymphoma: clinical advances and latest progress
Abstract
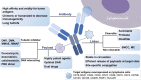
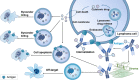
Antibody-drug conjugates (ADCs) are a promising class of immunotherapies with the potential to specifically target tumor cells and ameliorate the therapeutic index of cytotoxic drugs. ADCs comprise monoclonal antibodies, cytotoxic payloads with inherent antitumor activity, and specialized linkers connecting the two. In recent years, three ADCs, brentuximab vedotin, polatuzumab vedotin, and loncastuximab tesirine, have been approved and are already establishing their place in lymphoma treatment. As the efficacy and safety of ADCs have moved in synchrony with advances in their design, a plethora of novel ADCs have garnered growing interest as treatments. In this review, we provide an overview of the essential elements of ADC strategies in lymphoma and elucidate the up-to-date progress, current challenges, and novel targets of ADCs in this rapidly evolving field.
Keywords: Antibody-drug conjugates; Clinical trials; Immunotherapy; Lymphoma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, Damaj G, Gastinne T, Ribrag V, Feugier P, Casasnovas O, Zerazhi H, Haioun C, Maisonneuve H, Houot R, Jardin F, Van den Neste E, Tournilhac O, Le Du K, Morschhauser F, Cartron G, Fornecker LM, Canioni D, Callanan M, Bene MC, Salles G, Tilly H, Lamy T, Gressin R, Hermine O, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250–1260. doi: 10.1056/NEJMoa1701769. - DOI - PubMed
-
- Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trneny M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–1344. doi: 10.1056/NEJMoa1614598. - DOI - PubMed
-
- Thielen FW, Buyukkaramikli NC, Riemsma R, Fayter D, Armstrong N, Wei CY, Huertas Carrera V, Misso K, Worthy G, Kleijnen J, Corro Ramos I. Obinutuzumab in combination with chemotherapy for the first-line treatment of patients with advanced follicular lymphoma: an evidence review group evaluation of the NICE single technology appraisal. Pharmacoeconomics. 2019;37(8):975–984. doi: 10.1007/s40273-018-0740-4. - DOI - PMC - PubMed
-
- Goy A, Ramchandren R, Ghosh N, Munoz J, Morgan DS, Dang NH, Knapp M, Delioukina M, Kingsley E, Ping J, Beaupre DM, Neuenburg JK, Ruan J. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–1036. doi: 10.1182/blood.2018891598. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

