Circulating immune cell populations related to primary breast cancer, surgical removal, and radiotherapy revealed by flow cytometry analysis
- PMID: 34090509
- PMCID: PMC8180078
- DOI: 10.1186/s13058-021-01441-8
Circulating immune cell populations related to primary breast cancer, surgical removal, and radiotherapy revealed by flow cytometry analysis
Abstract
Background: Advanced breast cancer (BC) impact immune cells in the blood but whether such effects may reflect the presence of early BC and its therapeutic management remains elusive.
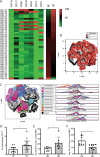
Methods: To address this question, we used multiparametric flow cytometry to analyze circulating leukocytes in patients with early BC (n = 13) at the time of diagnosis, after surgery, and after adjuvant radiotherapy, compared to healthy individuals. Data were analyzed using a minimally supervised approach based on FlowSOM algorithm and validated manually.
Results: At the time of diagnosis, BC patients have an increased frequency of CD117+CD11b+ granulocytes, which was significantly reduced after tumor removal. Adjuvant radiotherapy increased the frequency of CD45RO+ memory CD4+ T cells and CD4+ regulatory T cells. FlowSOM algorithm analysis revealed several unanticipated populations, including cells negative for all markers tested, CD11b+CD15low, CD3+CD4-CD8-, CD3+CD4+CD8+, and CD3+CD8+CD127+CD45RO+ cells, associated with BC or radiotherapy.
Conclusions: This study revealed changes in blood leukocytes associated with primary BC, surgical removal, and adjuvant radiotherapy. Specifically, it identified increased levels of CD117+ granulocytes, memory, and regulatory CD4+ T cells as potential biomarkers of BC and radiotherapy, respectively. Importantly, the study demonstrates the value of unsupervised analysis of complex flow cytometry data to unravel new cell populations of potential clinical relevance.
Keywords: Biomarker; Breast cancer; CD117; FlowJo; Granulocytes; Monocytes; Radiotherapy; Unsupervised analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge Ø, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

