IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis
- PMID: 34090510
- PMCID: PMC8180147
- DOI: 10.1186/s13287-021-02392-9
IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis
Abstract
Background: Ulcerative colitis (UC) is a chronic, relapsing, and non-specific inflammatory bowel disease, and the current treatment strategies were mainly used to relieve symptoms or for maintenance. Endometrial regenerative cells (ERCs) are mesenchymal-like stromal cells and have been demonstrated to alleviate multiple immune-dysregulation diseases. Pro-inflammatory stimuli were reported to enhance the immunosuppressive functions of ERCs, but the mechanism underlined is not fully understood. Here, we have designed this study to investigate the therapeutic effects of IL-1β-primed ERCs in the attenuation of experimental colitis.
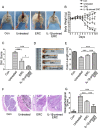
Methods: BALB/c mice were given 3% dextran sodium sulfate (DSS) for 7 consecutive days and free tap water for 3 days sequentially to induce experimental colitis. PBS (200 μL), ERCs, and IL-1β-primed ERCs (10ng/mL, 48 h) were injected (1 million/mouse/day, i.v.) on day 2, 5, and 8, respectively. Colonic and splenic samples were harvested on day 10 after DSS induction.
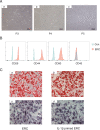
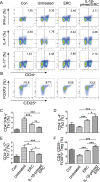
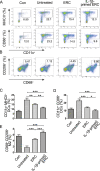
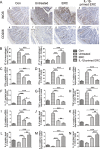
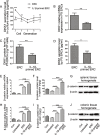
Results: It was found that IL-1β-primed ERC treatment markedly attenuated colonic damage, body weight loss, and colon length shortening in colitis mice. Compared with other treatments, cell populations of CD4+IL-4+Th2 cells, CD4+CD25+FOXP3+ regulatory T cells (Tregs), and CD68+CD206+ macrophages in spleens were also significantly upregulated in the IL-1β-primed ERC-treated group (p < 0.05). In addition, lower expression of pro-inflammatory (IFN-γ, IL-17, TNF-α, and IL-6), but higher levels of anti-inflammatory cytokines (IL-4 and IL-10) were detected in colons in the IL-1β-primed ERC-treated group (p < 0.05 vs. other groups). Importantly, we also found that different generations of ERCs had an overall lower secretion of Dickkopf-1 (DKK1) by IL-1β pre-stimulation (p < 0.05) and a higher expression of β-catenin in colonic and splenic tissues after the administration of IL-1β-primed ERCs.
Conclusions: This study has demonstrated that IL-1β pre-stimulation effectively downregulated DKK1 expression in ERCs, which in turn promoted the wnt/β-catenin pathway activation in colonic and splenic tissues. Consequently, IL-1β-primed ERCs exhibited an enhanced therapeutic effect in the attenuation of DSS-induced colitis.
Keywords: Colitis; Dickkopf-1; Endometrial regenerative cells; Immunoregulation; Mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice.J Transl Med. 2014 Dec 5;12:344. doi: 10.1186/s12967-014-0344-5. J Transl Med. 2014. PMID: 25475342 Free PMC article.
-
CD73 mediated host purinergic metabolism in intestine contributes to the therapeutic efficacy of a novel mesenchymal-like endometrial regenerative cells against experimental colitis.Front Immunol. 2023 Apr 25;14:1155090. doi: 10.3389/fimmu.2023.1155090. eCollection 2023. Front Immunol. 2023. PMID: 37180168 Free PMC article.
-
Melatonin pretreatment improves endometrial regenerative cell-mediated therapeutic effects in experimental colitis.Int Immunopharmacol. 2024 May 30;133:112092. doi: 10.1016/j.intimp.2024.112092. Epub 2024 Apr 15. Int Immunopharmacol. 2024. PMID: 38626548
-
The Cell-Specific Effects of JAK1 Inhibitors in Ulcerative Colitis.J Clin Med. 2025 Jan 18;14(2):608. doi: 10.3390/jcm14020608. J Clin Med. 2025. PMID: 39860613 Free PMC article. Review.
-
Human Endometrial Regenerative Cells for Neurological Disorders: Hype or Hope?Int J Stem Cells. 2024 Aug 30;17(3):224-235. doi: 10.15283/ijsc23091. Epub 2024 Jan 8. Int J Stem Cells. 2024. PMID: 38185531 Free PMC article. Review.
Cited by
-
A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways.Int J Mol Sci. 2025 Feb 21;26(5):1866. doi: 10.3390/ijms26051866. Int J Mol Sci. 2025. PMID: 40076493 Free PMC article.
-
Early weaning causes small intestinal atrophy by inhibiting the activity of intestinal stem cells: involvement of Wnt/β-catenin signaling.Stem Cell Res Ther. 2023 Apr 5;14(1):65. doi: 10.1186/s13287-023-03293-9. Stem Cell Res Ther. 2023. PMID: 37020258 Free PMC article.
-
Improving the Efficacy of Mesenchymal Stem/Stromal-Based Therapy for Treatment of Inflammatory Bowel Diseases.Biomedicines. 2021 Oct 20;9(11):1507. doi: 10.3390/biomedicines9111507. Biomedicines. 2021. PMID: 34829736 Free PMC article. Review.
-
Molecular regulation after mucosal injury and regeneration in ulcerative colitis.Front Mol Biosci. 2022 Oct 13;9:996057. doi: 10.3389/fmolb.2022.996057. eCollection 2022. Front Mol Biosci. 2022. PMID: 36310594 Free PMC article. Review.
-
Therapeutic Effect of Mesenchymal Stem Cells in Ulcerative Colitis: A Review on Achievements and Challenges.J Clin Med. 2020 Dec 3;9(12):3922. doi: 10.3390/jcm9123922. J Clin Med. 2020. PMID: 33287220 Free PMC article. Review.
References
-
- Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29(7):2727–2737. - PubMed
-
- Matsuoka K, Kobayashi T, Ueno F, Matsui T, Hirai F, Inoue N, Kato J, Kobayashi K, Kobayashi K, Koganei K, Kunisaki R, Motoya S, Nagahori M, Nakase H, Omata F, Saruta M, Watanabe T, Tanaka T, Kanai T, Noguchi Y, Takahashi KI, Watanabe K, Hibi T, Suzuki Y, Watanabe M, Sugano K, Shimosegawa T. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53(3):305–353. doi: 10.1007/s00535-018-1439-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

