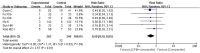Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials
- PMID: 34091029
- PMCID: PMC8180349
- DOI: 10.1016/j.ctim.2021.102744
Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials
Abstract
Introduction: Chinese patent medicine (CPM) is an indispensable part of traditional Chinese medicine. Coronavirus Disease 2019 (COVID-19) manifests is an acute respiratory infectious disease. This systematic review aimed to evaluate the therapeutic effects and safety of oral CPM for COVID-19.
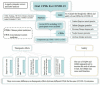
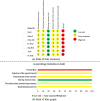
Methods: We included randomized controlled trials (RCTs) that tested oral CPM for the treatment of COVID-19 identified from publications in CNKI, Wanfang, VIP, Web of Science, SinoMed, PubMed, Embase, BioRxiv, MedRxiv and arXiv before November 2nd, 2020. The risk of bias for each trial was assessed using the Cochrane Risk of Bias Tool 2.0. RevMan 5.4 software was used for data analyses. The certainty of the evidence was assessed using the online GRADEpro tool.
Results: Seven RCTs including 1079 participants were identified. The overall bias was assessed as "-high risk of bias" for all included trials. Oral CPM investigated were: Lianhua Qingwen capsule/granules (LHQW), Jinhua Qinggan granules (JHQG), Huoxiang Zhengqi dripping pills (HXZQ), Toujie Quwen granules (TJQW) and Lianhua Qingke granules (LHQK). Compared with conventional western therapy alone for people with COVID-19: regarding the main outcomes, the results showed that oral CPM combined with conventional western therapy improved cure rate (RR = 1.20, 95 % CI 1.04-1.38, involving LHQW and TJQW), reduced aggravation rate (RR = 0.50, 95 % CI 0.29 - 0.85, involving LHQW, JHQG, LHQK and TJQW); with regard to additional outcomes, the results showed that add-on oral CPM shortened the duration of fever, cough and fatigue, improved the recovery rate of cough and fatigue, and increased the improvement and recovery rate of chest CT manifestations. There were some differences in therapeutic effects among various CPMs for the same COVID-19 outcome. The use of TJQW and LHQG appeared not to increase the risk of adverse events, but JHQG may cause mild diarrhea.
Conclusion: Low-certainty or very low-certainty evidence demonstrated that oral CPM may have add-on potential therapeutic effects for patients with non-serious COVID-19. These findings need to be further confirmed by well-designed clinical trials with adequate sample sizes.
Keywords: COVID-19; Chinese herbal medicine; Chinese patent medicine; Coronavirus Disease 2019; Meta-analysis; Systematic review.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization (WHO) 2020. Novel coronavirus – China.http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ Jan 12.
-
- World Health Organization (WHO) 2020. WHO Statement regarding cluster of pneumonia cases in Wuhan, China.https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding... Jan 9.
-
- World Health Organization (WHO) 2020. WHO Director-General’s remarks at the media briefing on 2019-nCo.https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at... Feb 11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical