Ligand conjugate SAR and enhanced delivery in NHP
- PMID: 34091052
- PMCID: PMC8531135
- DOI: 10.1016/j.ymthe.2021.06.002
Ligand conjugate SAR and enhanced delivery in NHP
Abstract
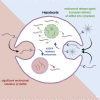
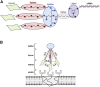
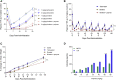
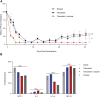
N-Acetylgalactosamine (GalNAc) conjugated short interfering RNAs (siRNAs) are a leading RNA interference (RNAi) platform allowing targeted inhibition of disease-causing genes in hepatocytes. More than a decade of development has recently resulted in the first approvals for this class of drugs. While substantial effort has been made to improve nucleic acid modification patterns for better payload stability and efficacy, relatively little attention has been given to the GalNAc targeting ligand. In addition, the lack of an intrinsic endosomal release mechanism has limited potency. Here, we report a stepwise analysis of the structure activity relationships (SAR) of the components comprising these targeting ligands. We show that there is relatively little difference in biological performance between bi-, tri-, and tetravalent ligand structures while identifying other features that affect their biological activity more significantly. Further, we demonstrate that subcutaneous co-administration of a GalNAc-functionalized, pH responsive endosomal release agent markedly improved the activity and duration of effect for siRNA conjugates, without compromising tolerability, in non-human primates. These findings could address a significant bottleneck for future siRNA ligand conjugate development.
Keywords: ASGPr; GalNAc; endosomal release; siRNA.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors are employees or consultants of Genevant Sciences Corporation or Arbutus Biopharma Corporation as noted in the author affiliations and own shares or stock options in their respective companies.
Figures
Comment in
-
Escaping to silence using an endosome-disrupting polymer.Mol Ther. 2021 Oct 6;29(10):2893-2894. doi: 10.1016/j.ymthe.2021.09.006. Epub 2021 Sep 23. Mol Ther. 2021. PMID: 34555311 Free PMC article. No abstract available.
References
-
- Second RNAi drug approved. (2020). Nat Biotechnol 38, 385. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

