Gsα-dependent signaling is required for postnatal establishment of a functional β-cell mass
- PMID: 34091063
- PMCID: PMC8239471
- DOI: 10.1016/j.molmet.2021.101264
Gsα-dependent signaling is required for postnatal establishment of a functional β-cell mass
Abstract
Objective: Early postnatal life is a critical period for the establishment of the functional β-cell mass that will sustain whole-body glucose homeostasis during the lifetime. β cells are formed from progenitors during embryonic development but undergo significant expansion in quantity and attain functional maturity after birth. The signals and pathways involved in these processes are not fully elucidated. Cyclic adenosine monophosphate (cAMP) is an intracellular signaling molecule that is known to regulate insulin secretion, gene expression, proliferation, and survival of adult β cells. The heterotrimeric G protein Gs stimulates the cAMP-dependent pathway by activating adenylyl cyclase. In this study, we sought to explore the role of Gs-dependent signaling in postnatal β-cell development.
Methods: To study Gs-dependent signaling, we generated conditional knockout mice in which the α subunit of the Gs protein (Gsα) was ablated from β-cells using the Cre deleter line Ins1Cre. Mice were characterized in terms of glucose homeostasis, including in vivo glucose tolerance, glucose-induced insulin secretion, and insulin sensitivity. β-cell mass was studied using histomorphometric analysis and optical projection tomography. β-cell proliferation was studied by ki67 and phospho-histone H3 immunostatining, and apoptosis was assessed by TUNEL assay. Gene expression was determined in isolated islets and sorted β cells by qPCR. Intracellular cAMP was studied in isolated islets using HTRF-based technology. The activation status of the cAMP and insulin-signaling pathways was determined by immunoblot analysis of the relevant components of these pathways in isolated islets. In vitro proliferation of dissociated islet cells was assessed by BrdU incorporation.
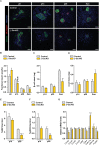
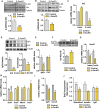
Results: Elimination of Gsα in β cells led to reduced β-cell mass, deficient insulin secretion, and severe glucose intolerance. These defects were evident by weaning and were associated with decreased proliferation and inadequate expression of key β-cell identity and maturation genes in postnatal β-cells. Additionally, loss of Gsα caused a broad multilevel disruption of the insulin transduction pathway that resulted in the specific abrogation of the islet proliferative response to insulin.
Conclusion: We conclude that Gsα is required for β-cell growth and maturation in the early postnatal stage and propose that this is partly mediated via its crosstalk with insulin signaling. Our findings disclose a tight connection between these two pathways in postnatal β cells, which may have implications for using cAMP-raising agents to promote β-cell regeneration and maturation in diabetes.
Keywords: Cell maturation; Gs; Insulin signaling; Postnatal development; Replication; cAMP; β-Cell mass.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Butler P.C., Meier J.J., Butler A.E., Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nature Clinical Practice Endocrinology and Metabolism. 2007;3(11):758–768. - PubMed
-
- Bouwens L., Rooman I. Regulation of pancreatic beta-cell mass. Physiological Reviews. 2005;85(4):1255–1270. - PubMed
-
- Wang Y., Sun J., Ni Q., Nie A., Gu Y. Dual effect of raptor on neonatal β-cell proliferation and identity maintenance. Diabetes. 2019;68(10):1950–1964. - PubMed
-
- Wang P., Fiaschi-Taesch N.M., Vasavada R.C., Scott D.K., Garcia-Ocana A., Stewart A.F. Diabetes mellitus--advances and challenges in human beta-cell proliferation. Nature Reviews Endocrinology. 2015;11(4):201–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

