Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells
- PMID: 34091442
- PMCID: PMC8202889
- DOI: 10.18632/aging.203068
Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells
Abstract
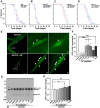
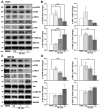
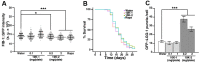
The medicinal fungus Ganoderma lucidum is used as a dietary supplement and health tonic, but whether it affects longevity remains unclear. We show here that a water extract of G. lucidum mycelium extends lifespan of the nematode Caenorhabditis elegans. The G. lucidum extract reduces the level of fibrillarin (FIB-1), a nucleolar protein that correlates inversely with longevity in various organisms. Furthermore, G. lucidum treatment increases expression of the autophagosomal protein marker LGG-1, and lifespan extension is abrogated in mutant C. elegans strains that lack atg-18, daf-16, or sir-2.1, indicating that autophagy and stress resistance pathways are required to extend lifespan. In cultured human cells, G. lucidum increases concentrations of the LGG-1 ortholog LC3 and reduces levels of phosphorylated mTOR, a known inhibitor of autophagy. Notably, low molecular weight compounds (<10 kDa) isolated from the G. lucidum water extract prolong lifespan of C. elegans and the same compounds induce autophagy in human cells. These results suggest that G. lucidum can increase longevity by inducing autophagy and stress resistance.
Keywords: caloric restriction mimetics; dietary supplements; lingzhi; mTOR; medicinal mushrooms.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

