Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder
- PMID: 34091591
- PMCID: PMC8179928
- DOI: 10.1038/s41398-021-01473-w
Genetic variations influence brain changes in patients with attention-deficit hyperactivity disorder
Abstract
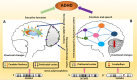
Attention-deficit hyperactivity disorder (ADHD) is a neurological and neurodevelopmental childhood-onset disorder characterized by a persistent pattern of inattentiveness, impulsiveness, restlessness, and hyperactivity. These symptoms may continue in 55-66% of cases from childhood into adulthood. Even though the precise etiology of ADHD is not fully understood, it is considered as a multifactorial and heterogeneous disorder with several contributing factors such as heritability, auxiliary to neurodevelopmental issues, severe brain injuries, neuroinflammation, consanguineous marriages, premature birth, and exposure to environmental toxins. Neuroimaging and neurodevelopmental assessments may help to explore the possible role of genetic variations on ADHD neuropsychobiology. Multiple genetic studies have observed a strong genetic association with various aspects of neuropsychobiological functions, including neural abnormalities and delayed neurodevelopment in ADHD. The advancement in neuroimaging and molecular genomics offers the opportunity to analyze the impact of genetic variations alongside its dysregulated pathways on structural and functional derived brain imaging phenotypes in various neurological and psychiatric disorders, including ADHD. Recently, neuroimaging genomic studies observed a significant association of brain imaging phenotypes with genetic susceptibility in ADHD. Integrating the neuroimaging-derived phenotypes with genomics deciphers various neurobiological pathways that can be leveraged for the development of novel clinical biomarkers, new treatment modalities as well as therapeutic interventions for ADHD patients. In this review, we discuss the neurobiology of ADHD with particular emphasis on structural and functional changes in the ADHD brain and their interactions with complex genomic variations utilizing imaging genetics methodologies. We also highlight the genetic variants supposedly allied with the development of ADHD and how these, in turn, may affect the brain circuit function and related behaviors. In addition to reviewing imaging genetic studies, we also examine the need for complementary approaches at various levels of biological complexity and emphasize the importance of combining and integrating results to explore biological pathways involved in ADHD disorder. These approaches include animal models, computational biology, bioinformatics analyses, and multimodal imaging genetics studies.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

