Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis
- PMID: 34091657
- PMCID: PMC8408869
- DOI: 10.1093/ajcn/nqab165
Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis
Abstract
Background: Prenatal alcohol exposure (PAE) is associated with postnatal iron deficiency (ID), which has been shown to exacerbate deficits in growth, cognition, and behavior seen in fetal alcohol spectrum disorders. However, the mechanisms underlying PAE-related ID remain unknown.
Objectives: We aimed to examine biochemical measures of iron homeostasis in the mother, placenta, neonate, and 6.5-month-old infant.
Methods: In a prenatally recruited, prospective longitudinal birth cohort in South Africa, 206 gravidas (126 heavy drinkers and 80 controls) were interviewed regarding alcohol, cigarette, and drug use and diet at 3 prenatal visits. Hemoglobin, ferritin, and soluble transferrin receptor (sTfR) were assayed twice during pregnancy and urinary hepcidin:creatinine was assayed once. Infant ferritin and hemoglobin were measured at 2 weeks and 6.5 months and sTfR was measured at 6.5 months. Histopathological examinations were conducted on 125 placentas and iron transport assays (iron regulatory protein-2, transferrin receptor-1, divalent metal transporter-1, ferroportin-1, and iron concentrations) were conducted on 63.
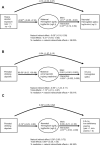
Results: In multivariable regression models, prenatal drinking frequency (days/week) was related to higher maternal hepcidin and to sequestration of iron into storage at the expense of erythropoiesis in mothers and neonates, as evidenced by a lower hemoglobin (g/dL)-to-log(ferritin) (ug/L) ratio [mothers: raw regression coefficient (β) = -0.21 (95% CI: -0.35 to -0.07); neonates: β = -0.15 (95% CI: -0.24 to -0.06)]. Drinking frequency was also related to decreased placental ferroportin-1:transferrin receptor-1 (β = -0.57 for logged values; 95% CI: -1.03 to -0.10), indicating iron-restricted placental iron transport. At 6.5 months, drinking frequency was associated with lower hemoglobin (β = -0.18; 95% CI: -0.33 to -0.02), and increased prevalences of ID (β = 0.09; 95% CI: 0.02-0.17) and ID anemia (IDA) (β = 0.13; 95% CI: 0.04-0.23). In causal inference analyses, the PAE-related increase in IDA was partially mediated by decreased neonatal hemoglobin:log(ferritin), and the decrease in neonatal hemoglobin:log(ferritin) was partially mediated by decreased maternal hemoglobin:log(ferritin).
Conclusions: In this study, greater PAE was associated with an unfavorable profile of maternal-fetal iron homeostasis, which may play mechanistic roles in PAE-related ID later in infancy.
Keywords: FAS; FASD; ID; IDA; fetal alcohol spectrum disorders; fetal alcohol syndrome; iron deficiency; iron deficiency anemia; placenta; prenatal alcohol exposure.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
References
-
- Carter RC, Wainwright H, Molteno CD, Georgieff MK, Dodge NC, Warton F, Meintjes EM, Jacobson JL, Jacobson SW. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res. 2016;40(4):753–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

