Transmission of cell-associated human cytomegalovirus isolates between various cell types using polymorphonuclear leukocytes as a vehicle
- PMID: 34091753
- PMCID: PMC8286230
- DOI: 10.1007/s00430-021-00713-6
Transmission of cell-associated human cytomegalovirus isolates between various cell types using polymorphonuclear leukocytes as a vehicle
Abstract
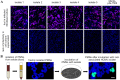
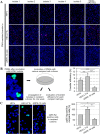
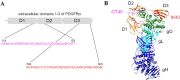
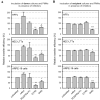
Polymorphonuclear leukocytes (PMNs) are regarded as vehicles for the hematogenous dissemination of human cytomegalovirus (HCMV). In cell culture, this concept has been validated with cell-free laboratory strains but not yet with clinical HCMV isolates that grow strictly cell-associated. We, therefore, aimed to evaluate whether PMNs can also transmit such isolates from initially infected fibroblasts to other cell types, which might further clarify the role of PMNs in HCMV dissemination and provide a model to search for potential inhibitors. PMNs, which have been isolated from HCMV-seronegative individuals, were added for 3 h to fibroblasts infected with recent cell-associated HCMV isolates, then removed and transferred to various recipient cell cultures. The transfer efficiency in the recipient cultures was evaluated by immunofluorescence staining of viral immediate early antigens. Soluble derivatives of the cellular HCMV entry receptor PDGFRα were analyzed for their potential to interfere with this transfer. All of five tested HCMV isolates could be transferred to fibroblasts, endothelial and epithelial cells with transfer rates ranging from 2 to 9%, and the transferred viruses could spread focally in these recipient cells within 1 week. The PDGFRα-derived peptides IK40 and GT40 reduced transfer by 40 and 70% when added during the uptake step. However, when added during the transfer step, only IK40 was effective, inhibiting transmission by 20% on endothelial cells and 50-60% on epithelial cells and fibroblasts. These findings further corroborate the assumption of cell-associated HCMV dissemination by PMNs and demonstrate that it is possible to inhibit this transmission mode.
Keywords: Cell-associated spread; Clinical isolates; HCMV; Hematogenous dissemination; PDGFRα-peptides; PMNs.
© 2021. The Author(s).
Conflict of interest statement
Aspects of this work are the subject of the patent filing WO2018002081A1.
Figures


Similar articles
-
Peptide Derivatives of Platelet-Derived Growth Factor Receptor Alpha Inhibit Cell-Associated Spread of Human Cytomegalovirus.Viruses. 2021 Sep 6;13(9):1780. doi: 10.3390/v13091780. Viruses. 2021. PMID: 34578361 Free PMC article.
-
A derivative of platelet-derived growth factor receptor alpha binds to the trimer of human cytomegalovirus and inhibits entry into fibroblasts and endothelial cells.PLoS Pathog. 2017 Apr 12;13(4):e1006273. doi: 10.1371/journal.ppat.1006273. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28403220 Free PMC article.
-
Selection of Human Cytomegalovirus Mutants with Resistance against PDGFRα-Derived Entry Inhibitors.Viruses. 2021 Jun 8;13(6):1094. doi: 10.3390/v13061094. Viruses. 2021. PMID: 34201364 Free PMC article.
-
Pathogenesis of human cytomegalovirus infection and cellular targets.Hum Immunol. 2004 May;65(5):381-6. doi: 10.1016/j.humimm.2004.02.009. Hum Immunol. 2004. PMID: 15172435 Review.
-
Human cytomegalovirus tropism for endothelial/epithelial cells: scientific background and clinical implications.Rev Med Virol. 2010 May;20(3):136-55. doi: 10.1002/rmv.645. Rev Med Virol. 2010. PMID: 20084641 Review.
Cited by
-
Peptide Derivatives of Platelet-Derived Growth Factor Receptor Alpha Inhibit Cell-Associated Spread of Human Cytomegalovirus.Viruses. 2021 Sep 6;13(9):1780. doi: 10.3390/v13091780. Viruses. 2021. PMID: 34578361 Free PMC article.
-
Viral and Cellular Factors Contributing to the Hematogenous Dissemination of Human Cytomegalovirus via Polymorphonuclear Leukocytes.Viruses. 2022 Jul 18;14(7):1561. doi: 10.3390/v14071561. Viruses. 2022. PMID: 35891541 Free PMC article.
-
Human cytomegalovirus and neonatal infection.Curr Res Microb Sci. 2024 Jun 24;7:100257. doi: 10.1016/j.crmicr.2024.100257. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39070527 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

