Prioritisation and design of clinical trials
- PMID: 34091766
- PMCID: PMC8629779
- DOI: 10.1007/s10654-021-00761-5
Prioritisation and design of clinical trials
Abstract
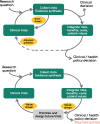
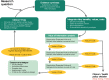
Clinical trials require participation of numerous patients, enormous research resources and substantial public funding. Time-consuming trials lead to delayed implementation of beneficial interventions and to reduced benefit to patients. This manuscript discusses two methods for the allocation of research resources and reviews a framework for prioritisation and design of clinical trials. The traditional error-driven approach of clinical trial design controls for type I and II errors. However, controlling for those statistical errors has limited relevance to policy makers. Therefore, this error-driven approach can be inefficient, waste research resources and lead to research with limited impact on daily practice. The novel value-driven approach assesses the currently available evidence and focuses on designing clinical trials that directly inform policy and treatment decisions. Estimating the net value of collecting further information, prior to undertaking a trial, informs a decision maker whether a clinical or health policy decision can be made with current information or if collection of extra evidence is justified. Additionally, estimating the net value of new information guides study design, data collection choices, and sample size estimation. The value-driven approach ensures the efficient use of research resources, reduces unnecessary burden to trial participants, and accelerates implementation of beneficial healthcare interventions.
Keywords: Clinical trial design; Research resources; Type I and type II errors; Uncertainty; Value of information analysis; Value-driven research.
© 2021. The Author(s).
Conflict of interest statement
No conflict of interest.
Figures
References
-
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;274(4):86–89. - PubMed
-
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ. 1996;5(6):513–524. - PubMed
-
- Conti S, Claxton K. Dimensions of design space: a decision-theoretic approach to optimal research design. Med Decis Mak. 2009;29(6):643–660. - PubMed
-
- Fleurence RL, Torgerson DJ. Setting priorities for research. Health Policy. 2004;69(1):1–10. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

