A review of nasopharyngeal swab and saliva tests for SARS-CoV-2 infection: Disease timelines, relative sensitivities, and test optimization
- PMID: 34091905
- PMCID: PMC8242677
- DOI: 10.1002/jso.26561
A review of nasopharyngeal swab and saliva tests for SARS-CoV-2 infection: Disease timelines, relative sensitivities, and test optimization
Abstract
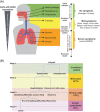
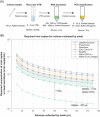
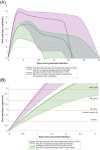
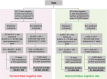
Testing is an essential part of containment of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. This review summarizes studies for SARS-CoV-2 infection and testing. Nasopharyngeal samples are best at sensitivity detection, especially in early stages of disease and in asymptomatic individuals. Current swab processing involves a 100- to 1000-fold dilution of the patient sample. Future optimization of testing should focus on using smaller volumes of viral transport media and swab designs to increase comfort and increased viral adhesion.
Keywords: COVID-19; bronchitis; pharyngitis; pneumonia; respiratory infection.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

References
-
- Allen JAS, Aufrichtig A, Barnard A, et al. Coronavirus World Map: Tracking the Global Outbreak. 2020. https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html
-
- CDC COVID Data Tracker 2020. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
-
- Coronavirus disease (COVID‐19): World Health Organization . 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question...
-
- Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature. 2020;581(7806):22‐26. - PubMed
-
- Burmer G, Burmer M, Pabuwal V. SARS‐CoV‐2 and COVID‐19 Pathogenesis: A Review. Seattle, WA: LifeSpan BioSciences, Inc.; 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

