The effect of immune cell-derived exosomes in the cardiac tissue repair after myocardial infarction: Molecular mechanisms and pre-clinical evidence
- PMID: 34092017
- PMCID: PMC8278122
- DOI: 10.1111/jcmm.16686
The effect of immune cell-derived exosomes in the cardiac tissue repair after myocardial infarction: Molecular mechanisms and pre-clinical evidence
Abstract
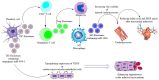
After a myocardial infarction (MI), the inflammatory responses are induced and assist to repair ischaemic injury and restore tissue integrity, but excessive inflammatory processes promote abnormal cardiac remodelling and progress towards heart failure. Thus, a timely resolution of inflammation and a firmly regulated balance between regulatory and inflammatory mechanisms can be helpful. Molecular- and cellular-based approaches modulating immune response post-MI have emerged as a promising therapeutic strategy. Exosomes are essential mediators of cell-to-cell communications, which are effective in modulating immune responses and immune cells following MI, improving the repair process of infarcted myocardium and maintaining ventricular function via the crosstalk among immune cells or between immune cells and myocardial cells. The present review aimed to seek the role of immune cell-secreted exosomes in infarcted myocardium post-MI, together with mechanisms behind their repairing impact on the damaged myocardium. The exosomes we focus on are secreted by classic immune cells including macrophages, dendritic cells, regulatory T cells and CD4+ T cells; however, further research is demanded to determine the role of exosomes secreted by other immune cells, such as B cells, neutrophils and mast cells, in infarcted myocardium after MI. This knowledge can assist in the development of future therapeutic strategies, which may benefit MI patients.
Keywords: cardiomyocyte; exosome; immune cell; inflammation; myocardial infarction.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest and financial support for the present review article.
Figures
Similar articles
-
Exosomes Induce Crosstalk Between Multiple Types of Cells and Cardiac Fibroblasts: Therapeutic Potential for Remodeling After Myocardial Infarction.Int J Nanomedicine. 2024 Oct 19;19:10605-10621. doi: 10.2147/IJN.S476995. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39445157 Free PMC article. Review.
-
Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization.Circulation. 2017 Apr 11;135(15):1444-1457. doi: 10.1161/CIRCULATIONAHA.116.023106. Epub 2017 Feb 7. Circulation. 2017. PMID: 28174192
-
Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction.J Mol Cell Cardiol. 2016 Feb;91:123-33. doi: 10.1016/j.yjmcc.2015.12.028. Epub 2015 Dec 30. J Mol Cell Cardiol. 2016. PMID: 26746143
-
C-X-C Motif Chemokine Receptor 4 Blockade Promotes Tissue Repair After Myocardial Infarction by Enhancing Regulatory T Cell Mobilization and Immune-Regulatory Function.Circulation. 2019 Apr 9;139(15):1798-1812. doi: 10.1161/CIRCULATIONAHA.118.036053. Circulation. 2019. PMID: 30696265 Free PMC article.
-
Immunomodulation by Exosomes in Myocardial Infarction.J Cardiovasc Transl Res. 2019 Feb;12(1):28-36. doi: 10.1007/s12265-018-9836-7. Epub 2018 Oct 29. J Cardiovasc Transl Res. 2019. PMID: 30374796 Review.
Cited by
-
Macrophages in myocardial infarction.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C1304-C1324. doi: 10.1152/ajpcell.00230.2022. Epub 2022 Sep 12. Am J Physiol Cell Physiol. 2022. PMID: 36094436 Free PMC article. Review.
-
Exosomes Induce Crosstalk Between Multiple Types of Cells and Cardiac Fibroblasts: Therapeutic Potential for Remodeling After Myocardial Infarction.Int J Nanomedicine. 2024 Oct 19;19:10605-10621. doi: 10.2147/IJN.S476995. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39445157 Free PMC article. Review.
-
Immune in myocardial ischemia/reperfusion injury: potential mechanisms and therapeutic strategies.Front Immunol. 2025 May 8;16:1558484. doi: 10.3389/fimmu.2025.1558484. eCollection 2025. Front Immunol. 2025. PMID: 40406107 Free PMC article. Review.
-
Cardiac Telocytes 16 Years on-What Have We Learned So Far, and How Close Are We to Routine Application of the Knowledge in Cardiovascular Regenerative Medicine?Int J Mol Sci. 2021 Oct 10;22(20):10942. doi: 10.3390/ijms222010942. Int J Mol Sci. 2021. PMID: 34681601 Free PMC article. Review.
-
Immune cell dynamics in heart failure: implicated mechanisms and therapeutic targets.ESC Heart Fail. 2025 Jun;12(3):1739-1758. doi: 10.1002/ehf2.15238. Epub 2025 Feb 4. ESC Heart Fail. 2025. PMID: 39905753 Free PMC article. Review.
References
-
- Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254‐e743. - PubMed
-
- Arslan F, De Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8(5):292. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials