T cell-mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19
- PMID: 34092033
- PMCID: PMC8222887
- DOI: 10.1111/ajt.16708
T cell-mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19
Abstract
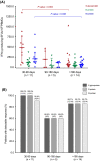
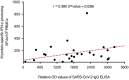
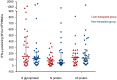
Whether immunosuppression impairs severe acute respiratory syndrome coronavirus 2-specific T cell-mediated immunity (SARS-CoV-2-CMI) after liver transplantation (LT) remains unknown. We included 31 LT recipients in whom SARS-CoV-2-CMI was assessed by intracellular cytokine staining (ICS) and interferon (IFN)-γ FluoroSpot assay after a median of 103 days from COVID-19 diagnosis. Serum SARS-CoV-2 IgG antibodies were measured by ELISA. A control group of nontransplant immunocompetent patients were matched (1:1 ratio) by age and time from diagnosis. Post-transplant SARS-CoV-2-CMI was detected by ICS in 90.3% (28/31) of recipients, with higher proportions for IFN-γ-producing CD4+ than CD8+ responses (93.5% versus 83.9%). Positive spike-specific and nucleoprotein-specific responses were found by FluoroSpot in 86.7% (26/30) of recipients each, whereas membrane protein-specific response was present in 83.3% (25/30). An inverse correlation was observed between the number of spike-specific IFN-γ-producing SFUs and time from diagnosis (Spearman's rho: -0.418; p value = .024). Two recipients (6.5%) failed to mount either T cell-mediated or IgG responses. There were no significant differences between LT recipients and nontransplant patients in the magnitude of responses by FluoroSpot to any of the antigens. Most LT recipients mount detectable-but declining over time-SARS-CoV-2-CMI after a median of 3 months from COVID-19, with no meaningful differences with immunocompetent patients.
Keywords: clinical research/practice; complication: infectious; immunosuppression/immune modulation; infection and infectious agents; infection and infectious agents - viral; infectious disease; liver transplantation/hepatology; monitoring: immune.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

