Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients With Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131
- PMID: 34092112
- PMCID: PMC8577688
- DOI: 10.1200/JCO.21.00976
Randomized Phase III Postoperative Trial of Platinum-Based Chemotherapy Versus Capecitabine in Patients With Residual Triple-Negative Breast Cancer Following Neoadjuvant Chemotherapy: ECOG-ACRIN EA1131
Abstract
Purpose: Patients with triple-negative breast cancer (TNBC) and residual invasive disease (RD) after completion of neoadjuvant chemotherapy (NAC) have a high-risk for recurrence, which is reduced by adjuvant capecitabine. Preclinical models support the use of platinum agents in the TNBC basal subtype. The EA1131 trial hypothesized that invasive disease-free survival (iDFS) would not be inferior but improved in patients with basal subtype TNBC treated with adjuvant platinum compared with capecitabine.
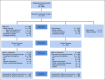
Patients and methods: Patients with clinical stage II or III TNBC with ≥ 1 cm RD in the breast post-NAC were randomly assigned to receive platinum (carboplatin or cisplatin) once every 3 weeks for four cycles or capecitabine 14 out of 21 days every 3 weeks for six cycles. TNBC subtype (basal v nonbasal) was determined by PAM50 in the residual disease. A noninferiority design with superiority alternative was chosen, assuming a 4-year iDFS of 67% with capecitabine.
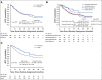
Results: Four hundred ten of planned 775 participants were randomly assigned to platinum or capecitabine between 2015 and 2021. After median follow-up of 20 months and 120 iDFS events (61% of full information) in the 308 (78%) patients with basal subtype TNBC, the 3-year iDFS for platinum was 42% (95% CI, 30 to 53) versus 49% (95% CI, 39 to 59) for capecitabine. Grade 3 and 4 toxicities were more common with platinum agents. The Data and Safety Monitoring Committee recommended stopping the trial as it was unlikely that further follow-up would show noninferiority or superiority of platinum.
Conclusion: Platinum agents do not improve outcomes in patients with basal subtype TNBC RD post-NAC and are associated with more severe toxicity when compared with capecitabine. Participants had a lower than expected 3-year iDFS regardless of study treatment, highlighting the need for better therapies in this high-risk population.
Trial registration: ClinicalTrials.gov NCT02445391.
Conflict of interest statement
Figures


Comment in
-
Critical Appraisal of Adjuvant Platinum-Based Chemotherapy for Basal Subtype Triple-Negative Breast Cancer With Residual Disease After Neoadjuvant Chemotherapy.J Clin Oncol. 2021 Nov 1;39(31):3519-3521. doi: 10.1200/JCO.21.01537. Epub 2021 Sep 23. J Clin Oncol. 2021. PMID: 34554822 No abstract available.
-
Reply to T. Shimoi et al and Y. Shimanuki et al.J Clin Oncol. 2021 Nov 1;39(31):3522-3524. doi: 10.1200/JCO.21.01905. Epub 2021 Sep 23. J Clin Oncol. 2021. PMID: 34554848 Free PMC article. No abstract available.
-
Are Platinum Drugs Ineffective for Triple-Negative Breast Cancer With Residual Invasive Disease After Neoadjuvant Chemotherapy?J Clin Oncol. 2021 Nov 1;39(31):3521-3522. doi: 10.1200/JCO.21.01757. Epub 2021 Sep 23. J Clin Oncol. 2021. PMID: 34554850 No abstract available.
Similar articles
-
OptimICE-RD: sacituzumab govitecan + pembrolizumab vs pembrolizumab (± capecitabine) for residual triple-negative breast cancer.Future Oncol. 2024;20(31):2343-2355. doi: 10.1080/14796694.2024.2357534. Epub 2024 Jun 26. Future Oncol. 2024. PMID: 38922307 Free PMC article.
-
Randomized, phase II trial to evaluate the efficacy and safety of atezolizumab plus capecitabine adjuvant therapy compared to capecitabine monotherapy for triple receptor-negative breast cancer with residual invasive cancer after neoadjuvant chemotherapy (MIRINAE trial, KCSG-BR18-21).BMC Cancer. 2025 Aug 9;25(1):1295. doi: 10.1186/s12885-025-14673-0. BMC Cancer. 2025. PMID: 40783751 Free PMC article. Clinical Trial.
-
Phase III Trial of Adjuvant Capecitabine After Standard Neo-/Adjuvant Chemotherapy in Patients With Early Triple-Negative Breast Cancer (GEICAM/2003-11_CIBOMA/2004-01).J Clin Oncol. 2020 Jan 20;38(3):203-213. doi: 10.1200/JCO.19.00904. Epub 2019 Dec 5. J Clin Oncol. 2020. PMID: 31804894 Free PMC article. Clinical Trial.
-
The role of capecitabine-based neoadjuvant and adjuvant chemotherapy in early-stage triple-negative breast cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Jan 19;21(1):78. doi: 10.1186/s12885-021-07791-y. BMC Cancer. 2021. PMID: 33468087 Free PMC article.
-
Platinum chemotherapy for early triple-negative breast cancer.Breast. 2024 Jun;75:103712. doi: 10.1016/j.breast.2024.103712. Epub 2024 Mar 12. Breast. 2024. PMID: 38492276 Free PMC article.
Cited by
-
Role of High-Dose Adjuvant Chemotherapy Followed by Autologous Stem Cell Transplantation in Locally Advanced Triple-Negative Breast Cancer: A Retrospective Chart Review.J Oncol. 2022 Sep 30;2022:3472324. doi: 10.1155/2022/3472324. eCollection 2022. J Oncol. 2022. PMID: 36213836 Free PMC article.
-
Shifting the Paradigm: The Transformative Role of Neoadjuvant Therapy in Early Breast Cancer.Cancers (Basel). 2024 Sep 23;16(18):3236. doi: 10.3390/cancers16183236. Cancers (Basel). 2024. PMID: 39335206 Free PMC article. Review.
-
Adjuvant capecitabine in triple negative breast cancer patients with residual disease after neoadjuvant treatment: real-world evidence from CaRe, a multicentric, observational study.Front Oncol. 2023 May 16;13:1152123. doi: 10.3389/fonc.2023.1152123. eCollection 2023. Front Oncol. 2023. PMID: 37260975 Free PMC article.
-
Platinum-based chemotherapy for early triple-negative breast cancer.Cochrane Database Syst Rev. 2023 Sep 8;9(9):CD014805. doi: 10.1002/14651858.CD014805.pub2. Cochrane Database Syst Rev. 2023. PMID: 37681577 Free PMC article.
-
Are more courses of immunochemotherapy beneficial for the short-term outcome of locally advanced esophageal squamous cell carcinoma?Thorac Cancer. 2023 May;14(13):1153-1161. doi: 10.1111/1759-7714.14843. Epub 2023 Mar 24. Thorac Cancer. 2023. PMID: 36960736 Free PMC article.
References
-
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence Clin Cancer Res 134429–44342007 - PubMed
-
- Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study JAMA 2952492–25022006 - PubMed
-
- von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes J Clin Oncol 301796–18042012 - PubMed
-
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer J Clin Oncol 261275–12812008 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA189971/CA/NCI NIH HHS/United States
- UG1 CA233320/CA/NCI NIH HHS/United States
- UG1 CA189859/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- UG1 CA233302/CA/NCI NIH HHS/United States
- UG1 CA189863/CA/NCI NIH HHS/United States
- UG1 CA189809/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA239769/CA/NCI NIH HHS/United States
- UG1 CA189856/CA/NCI NIH HHS/United States
- UG1 CA233270/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- UG1 CA189851/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233277/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- UG1 CA189954/CA/NCI NIH HHS/United States
- UG1 CA233340/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

