Incidence of Device Associated-Healthcare Associated Infections from a Neurosurgical Intensive Care Unit of a Tertiary Care Center: A Retrospective Analysis
- PMID: 34092858
- PMCID: PMC8159032
- DOI: 10.4103/aer.AER_112_20
Incidence of Device Associated-Healthcare Associated Infections from a Neurosurgical Intensive Care Unit of a Tertiary Care Center: A Retrospective Analysis
Abstract
Background: Deviceassociated infections (DAIs) increase the morbidity and mortality in the intensive care unit (ICU). Studies from the neurosurgical ICU in developing countries are sparse.
Aims: The aim of this study was to assess the incidence of device-associated healthcare associated infections, pathogens isolated, antibiotic resistance, and mortality in neurosurgical ICU.
Settings and design: A retrospective study was conducted in the neurosurgical ICU of a tertiary care center.
Materials and methods: This study was done by analyzing data of patients admitted in a neurosurgical ICU with one or more devices during the period from January 2011 to July 2017.
Statistical analysis: Quantitative variables were expressed as mean and standard deviation; qualitative variables were expressed as frequency and percentage.
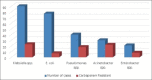
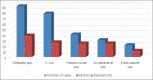
Results: During this period, 6788 patients with devices were admitted in the ICU, and 316 patients developed DAI. Two hundred and forty-eight patients had catheter-associated urinary tract infection (CAUTI), 78 had ventilator-associated pneumonia (VAP), and 53 had central line-associated bloodstream infection (CLABSI). The incidence rate for CAUTI was 17.83, VAP - 16.83, and CLABSI - 4.39 per 1000 device days. The device utilization ratio was highest for urinary catheter - 0.76, followed by central line - 0.66 and ventilator - 0.25. Predominant pathogens were Klebsiella - 90, Escherichia coli - 77, Pseudomonas - 40, Candida - 39, Acinetobacter - 30, and Enterobacter - 21. Carbapenem resistance was found in Acinetobacter (73.4%), Pseudomonas (45%), and Enterobacter (38%). S. aureus isolated in six cases; four being MRSA (66.7%). Multidrug resistance was found in Acinetobacter (80%), Pseudomonas (60%), Enterobacter (52.3%), Klebsiella (42.3%), and E. coli (33.7%). No colistin resistant Gram negative bacilli or vancomycin resistant enterococci were isolated. During this period 124 patients with DAI died, of which 52 patients had sepsis. The crude mortality rate was 1.83%.
Conclusion: The DAI with the highest incidence was CAUTI, followed by VAP and CLABSI. With the implementation of insertion bundles and adherence to aseptic precautions, the DAI rate had come down.
Keywords: Central line-associated blood stream infection; catheter-associated urinary tract infection; device-associated infection; healthcare-associated infection; neurosurgical intensive care unit; ventilator-associated pneumonia.
Copyright: © 2021 Anesthesia: Essays and Researches.
Conflict of interest statement
There are no conflicts of interest.
Figures



Similar articles
-
Catheter-Associated Urinary Tract Infection in Neurological Intensive Care Units: A Narrative Review.Neurohospitalist. 2022 Jul;12(3):484-497. doi: 10.1177/19418744221075888. Epub 2022 Feb 25. Neurohospitalist. 2022. PMID: 35755214 Free PMC article. Review.
-
Prospective surveillance of device-associated health care-associated infection in an intensive care unit of a tertiary care hospital in New Delhi, India.Am J Infect Control. 2018 Feb;46(2):202-206. doi: 10.1016/j.ajic.2017.08.037. Epub 2017 Oct 16. Am J Infect Control. 2018. PMID: 29046215
-
Morbidity, mortality, and emerging drug resistance in Device-associated infections (DAIs) in intensive care patients at a 1000-bedded tertiary care teaching hospital.Med J Armed Forces India. 2022 Apr;78(2):221-231. doi: 10.1016/j.mjafi.2021.06.031. Epub 2021 Oct 28. Med J Armed Forces India. 2022. PMID: 35463554 Free PMC article.
-
Epidemiology and Microbiological Profile of Common Healthcare Associated Infections among Patients in the Intensive Care Unit of a General Hospital in Kuwait: A Retrospective Observational Study.J Epidemiol Glob Health. 2021 Sep;11(3):302-309. doi: 10.2991/jegh.k.210524.001. Epub 2021 Jun 5. J Epidemiol Glob Health. 2021. PMID: 34270184 Free PMC article.
-
Evidence for the effectiveness of chlorhexidine bathing and health care-associated infections among adult intensive care patients: a trial sequential meta-analysis.BMC Infect Dis. 2018 Dec 19;18(1):679. doi: 10.1186/s12879-018-3521-y. BMC Infect Dis. 2018. PMID: 30567493 Free PMC article. Review.
Cited by
-
Catheter-Associated Urinary Tract Infection in Neurological Intensive Care Units: A Narrative Review.Neurohospitalist. 2022 Jul;12(3):484-497. doi: 10.1177/19418744221075888. Epub 2022 Feb 25. Neurohospitalist. 2022. PMID: 35755214 Free PMC article. Review.
References
-
- NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, issued August 2003. Am J Infect Control. 2003;31:481–98. - PubMed
-
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National nosocomial infections surveillance system. Crit Care Med. 1999;27:887–92. - PubMed
-
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598–601. - PubMed
-
- Stocchetti N, Rossi S, Zanier ER, Colombo A, Beretta L, Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28:1555–62. - PubMed
LinkOut - more resources
Full Text Sources
