Airborne virus transmission via respiratory droplets: Effects of droplet evaporation and sedimentation
- PMID: 34093064
- PMCID: PMC8164513
- DOI: 10.1016/j.cocis.2021.101471
Airborne virus transmission via respiratory droplets: Effects of droplet evaporation and sedimentation
Abstract
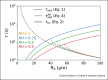
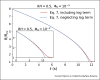
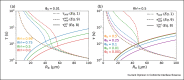
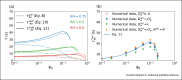
Airborne transmission is considered as an important route for the spread of infectious diseases, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and is primarily determined by the droplet sedimentation time, that is, the time droplets spend in air before reaching the ground. Evaporation increases the sedimentation time by reducing the droplet mass. In fact, small droplets can, depending on their solute content, almost completely evaporate during their descent to the ground and remain airborne as so-called droplet nuclei for a long time. Considering that viruses possibly remain infectious in aerosols for hours, droplet nuclei formation can substantially increase the infectious viral air load. Accordingly, the physical-chemical factors that control droplet evaporation and sedimentation times and play important roles in determining the infection risk from airborne respiratory droplets are reviewed in this article.
Keywords: Airborne virus transmission; Droplet evaporation; Droplet nuclei; Droplet sedimentation; Wells model.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Wells W.F. ON air-borne infection∗: study II. Droplets and droplet nuclei. Am J Epidemiol. 1934;20:611–618.
-
- Leung Nancy H.L., Chu Daniel K.W., Shiu Eunice Y.C., Chan Kwok-Hung, McDevitt James J., Hau Benien J.P., Yen Hui-Ling, Li Yuguo, Ip Dennis K.M., Malik Peiris JS, Seto Wing-Hong, Leung Gabriel M., Milton Donald K., Cowling Benjamin J. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. - PMC - PubMed
-
- Bridges C., Kuehnert M., Hall C. Transmission of influenza: implications for control in health care settings. Clin Infect Dis. 2003;37:1094–1101. - PubMed
-
- Klompas M., Baker M., Rhee C. Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. J Am Med Assoc. 2020;324 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
