Dexmedetomidine Protects Human Cardiomyocytes Against Ischemia-Reperfusion Injury Through α2-Adrenergic Receptor/AMPK-Dependent Autophagy
- PMID: 34093174
- PMCID: PMC8176440
- DOI: 10.3389/fphar.2021.615424
Dexmedetomidine Protects Human Cardiomyocytes Against Ischemia-Reperfusion Injury Through α2-Adrenergic Receptor/AMPK-Dependent Autophagy
Abstract
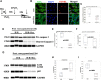
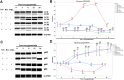
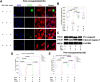
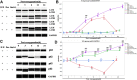
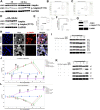
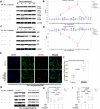
Background: Ischemia-reperfusion injury (I/R) strongly affects the prognosis of children with complicated congenital heart diseases (CHDs) who undergo long-term cardiac surgical processes. Recently, the α2-adrenergic receptor agonist Dexmedetomidine (Dex) has been reported to protect cardiomyocytes (CMs) from I/R in cellular models and adult rodent models. However, whether and how Dex may protect human CMs in young children remains largely unknown. Methods and Results: Human ventricular tissue from tetralogy of Fallot (TOF) patients and CMs derived from human-induced pluripotent stem cells (iPSC-CMs) were used to assess whether and how Dex protects human CMs from I/R. The results showed that when pretreated with Dex, the apoptosis marker-TUNEL and cleaved caspase 3 in the ventricular tissue were significantly reduced. In addition, the autophagy marker LC3II was significantly increased compared with that of the control group. When exposed to the hypoxia/reoxygenation process, iPSC-CMs pretreated with Dex also showed reduced TUNEL and cleaved caspase 3 and increased LC3II. When the autophagy inhibitor (3-methyladenine, 3-MA) was applied to the iPSC-CMs, the protective effect of Dex on the CMs was largely blocked. In addition, when the fusion of autophagosomes with lysosomes was blocked by Bafilomycin A1, the degradation of p62 induced by Dex during the autophagy process was suspended. Moreover, when pretreated with Dex, both the human ventricle and the iPSC-CMs expressed more AMP-activated protein kinase (AMPK) and phospho AMPK (pAMPK) during the I/R process. After AMPK knockout or the use of an α2-adrenergic receptor antagonist-yohimbine, the protection of Dex and its enhancement of autophagy were inhibited. Conclusion: Dex protects young human CMs from I/R injury, and α2-adrenergic receptor/AMPK-dependent autophagy plays an important role during this process. Dex may have a therapeutic effect for children with CHD who undergo long-term cardiac surgical processes.
Keywords: autophagy; cardiomyocyte; congenital heart disease; dexmedetomidine; ischemia-reperfusion injury.
Copyright © 2021 Xiao, Li, Qiu, Jiang, Huang, Liu, Sun, Hong and Ye.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dexmedetomidine Alleviates Ischemia/Reperfusion-Associated Acute Kidney Injury by Enhancing Autophagic Activity via the α2-AR/AMPK/mTOR Pathway.Front Biosci (Landmark Ed). 2023 Dec 1;28(12):323. doi: 10.31083/j.fbl2812323. Front Biosci (Landmark Ed). 2023. PMID: 38179733
-
Dexmedetomidine protects the heart against ischemia reperfusion injury via regulation of the bradykinin receptors.Eur J Pharmacol. 2021 Nov 15;911:174493. doi: 10.1016/j.ejphar.2021.174493. Epub 2021 Sep 8. Eur J Pharmacol. 2021. PMID: 34506777
-
Dexmedetomidine protects cardiac microvascular endothelial cells from the damage of ogd/r through regulation of the pparδ-mediated autophagy.Microcirculation. 2021 May;28(4):e12675. doi: 10.1111/micc.12675. Epub 2021 Mar 2. Microcirculation. 2021. PMID: 33377581
-
Amelioration of myocardial ischemia/reperfusion injury in diabetes: A narrative review of the mechanisms and clinical applications of dexmedetomidine.Front Pharmacol. 2022 Aug 31;13:949754. doi: 10.3389/fphar.2022.949754. eCollection 2022. Front Pharmacol. 2022. PMID: 36120296 Free PMC article. Review.
-
The neuroprotective effect of dexmedetomidine and its mechanism.Front Pharmacol. 2022 Sep 20;13:965661. doi: 10.3389/fphar.2022.965661. eCollection 2022. Front Pharmacol. 2022. PMID: 36204225 Free PMC article. Review.
Cited by
-
Dexmedetomidine improves acute lung injury by activating autophagy in a rat hemorrhagic shock and resuscitation model.Sci Rep. 2023 Mar 16;13(1):4374. doi: 10.1038/s41598-023-31483-1. Sci Rep. 2023. PMID: 36927753 Free PMC article.
-
Cardioprotective effects of the electrolyte solution sterofundin and the possible underlying mechanisms.Front Pharmacol. 2025 Jan 3;15:1449831. doi: 10.3389/fphar.2024.1449831. eCollection 2024. Front Pharmacol. 2025. PMID: 39830345 Free PMC article.
-
Targeting ferroptosis as a promising therapeutic strategy to treat cardiomyopathy.Front Pharmacol. 2023 Apr 13;14:1146651. doi: 10.3389/fphar.2023.1146651. eCollection 2023. Front Pharmacol. 2023. PMID: 37138856 Free PMC article. Review.
-
Dexmedetomidine combined with propofol attenuates myocardial ischemia/reperfusion injury by activating the AMPK signaling pathway.Heliyon. 2023 Nov 4;9(11):e22054. doi: 10.1016/j.heliyon.2023.e22054. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034796 Free PMC article.
-
Influence of Dexmedetomidine on Myocardial Injury in Patients with Simultaneous Pancreas-Kidney Transplantation.Evid Based Complement Alternat Med. 2022 Nov 17;2022:7196449. doi: 10.1155/2022/7196449. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Aug 30;2023:9860397. doi: 10.1155/2023/9860397. PMID: 36437830 Free PMC article. Retracted.
References
LinkOut - more resources
Full Text Sources
Research Materials

