The Role of Embryonic Chick Muscle Cell Culture in the Study of Skeletal Myogenesis
- PMID: 34093232
- PMCID: PMC8173222
- DOI: 10.3389/fphys.2021.668600
The Role of Embryonic Chick Muscle Cell Culture in the Study of Skeletal Myogenesis
Abstract
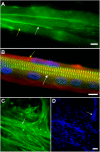
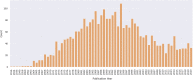
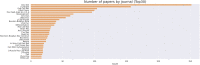
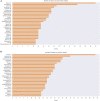
The mechanisms involved in the development of skeletal muscle fibers have been studied in the last 70 years and yet many aspects of this process are still not completely understood. A myriad of in vivo and in vitro invertebrate and vertebrate animal models has been used for dissecting the molecular and cellular events involved in muscle formation. Among the most used animal models for the study of myogenesis are the rodents rat and mouse, the fruit fly Drosophila, and the birds chicken and quail. Here, we describe the robustness and advantages of the chick primary muscle culture model for the study of skeletal myogenesis. In the myoblast culture obtained from embryonic chick pectoralis muscle it is possible to analyze all the steps involved in skeletal myogenesis, such as myoblast proliferation, withdrawal from cell cycle, cell elongation and migration, myoblast alignment and fusion, the assembly of striated myofibrils, and the formation of multinucleated myotubes. The fact that in vitro chick myotubes can harbor hundreds of nuclei, whereas myotubes from cell lines have only a dozen nuclei demonstrates the high level of differentiation of the autonomous chick myogenic program. This striking differentiation is independent of serum withdrawal, which points to the power of the model. We also review the major pro-myogenic and anti-myogenic molecules and signaling pathways involved in chick myogenesis, in addition to providing a detailed protocol for the preparation of embryonic chick myogenic cultures. Moreover, we performed a bibliometric analysis of the articles that used this model to evaluate which were the main explored topics of interest and their contributors. We expect that by describing the major findings, and their advantages, of the studies using the embryonic chick myogenic model we will foster new studies on the molecular and cellular process involved in muscle proliferation and differentiation that are more similar to the actual in vivo condition than the muscle cell lines.
Keywords: chick embryo; muscle differentiation; myoblast; myogenesis; myotube; skeletal muscle.
Copyright © 2021 Costa, Jurberg and Mermelstein.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Geldanamycin inhibits in vivo and in vitro chick skeletal myogenesis.Dev Biol. 2025 Jun;522:20-29. doi: 10.1016/j.ydbio.2025.02.018. Epub 2025 Mar 7. Dev Biol. 2025. PMID: 40058749
-
A conserved role for calpains during myoblast fusion.Genesis. 2015 Jul;53(7):417-30. doi: 10.1002/dvg.22870. Epub 2015 Jul 14. Genesis. 2015. PMID: 26138338
-
Rotenone inhibits embryonic chick myogenesis in a ROS-dependent mechanism.Tissue Cell. 2024 Aug;89:102423. doi: 10.1016/j.tice.2024.102423. Epub 2024 May 27. Tissue Cell. 2024. PMID: 38875923
-
Mechanisms regulating myoblast fusion: A multilevel interplay.Semin Cell Dev Biol. 2020 Aug;104:81-92. doi: 10.1016/j.semcdb.2020.02.004. Epub 2020 Feb 13. Semin Cell Dev Biol. 2020. PMID: 32063453 Review.
-
Regulation of Skeletal Muscle Myoblast Differentiation and Proliferation by Pannexins.Adv Exp Med Biol. 2017;925:57-73. doi: 10.1007/5584_2016_53. Adv Exp Med Biol. 2017. PMID: 27518505 Review.
Cited by
-
Expression patterns and correlation analyses of muscle-specific genes in the process of sheep myoblast differentiation.In Vitro Cell Dev Biol Anim. 2022 Oct;58(9):798-809. doi: 10.1007/s11626-022-00721-7. Epub 2022 Sep 30. In Vitro Cell Dev Biol Anim. 2022. PMID: 36178582
-
Molecular Pathways and Key Genes Associated With Breast Width and Protein Content in White Striping and Wooden Breast Chicken Pectoral Muscle.Front Physiol. 2022 Jul 8;13:936768. doi: 10.3389/fphys.2022.936768. eCollection 2022. Front Physiol. 2022. PMID: 35874513 Free PMC article.
-
Bulk and single-cell alternative splicing analyses reveal roles of TRA2B in myogenic differentiation.Cell Prolif. 2024 Feb;57(2):e13545. doi: 10.1111/cpr.13545. Epub 2023 Sep 13. Cell Prolif. 2024. PMID: 37705195 Free PMC article.
-
The Wnt/Beta-Catenin Pathway and Cytoskeletal Filaments Are Involved in the Positioning, Size, and Function of Lysosomes during Chick Myogenesis.Cells. 2022 Oct 27;11(21):3402. doi: 10.3390/cells11213402. Cells. 2022. PMID: 36359798 Free PMC article.
-
Transcriptome Sequencing Analysis of circRNA in Skeletal Muscle between Fast- and Slow-Growing Chickens at Embryonic Stages.Animals (Basel). 2022 Nov 16;12(22):3166. doi: 10.3390/ani12223166. Animals (Basel). 2022. PMID: 36428392 Free PMC article.
References
-
- Almenar-Queralt A., Gregorio C. C., Fowler V. M. (1999). Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112 1111–1123. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

