The Alteration of Salivary Immunoglobulin A in Autism Spectrum Disorders
- PMID: 34093280
- PMCID: PMC8175640
- DOI: 10.3389/fpsyt.2021.669193
The Alteration of Salivary Immunoglobulin A in Autism Spectrum Disorders
Abstract
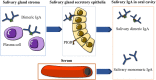
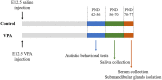
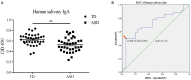
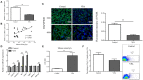
Objectives: Autism spectrum disorders (ASD) are neurodevelopmental disorders with changes in the gut and oral microbiota. Based on the intimate relationship between the oral microbiota and oral mucosal immunity, this study aimed to investigate changes in salivary immunoglobulin A (IgA) level in ASD and the underlying mechanism for any such changes. Methods: We recruited 36 children diagnosed with ASD and 35 normally developing children and measured their salivary IgA content using enzyme-linked immunosorbent assay (ELISA). The valproate (VPA) -treated ASD mouse model was established by prenatal exposure to valproate and mouse salivary IgA content was also quantified by ELISA. The submandibular glands of VPA and control mice were isolated and analyzed using qRT-PCR, immunofluorescence staining, and flow cytometry. ASD-related Streptococci were co-incubated with the human salivary gland (HSG) cell line, and western blotting was used to detect the levels of relevant proteins. Results: We found that salivary IgA content was significantly decreased in patients with ASD and had a significant ASD diagnostic value. The salivary IgA content also decreased in VPA mice and was significantly correlated with autistic-like behaviors among them. The mRNA and protein levels of the polymeric immunoglobulin receptor (Pigr) were downregulated in the submandibular glands of VPA mice and the Pigr mRNA level was positively correlated with mouse salivary IgA content. HSG cells treated with ASD-related Streptococci had reduced PIGR protein level. Conclusion: Therefore, protective IgA levels were reduced in the saliva of individuals with ASD, which correlated with the bacteria-induced downregulation of Pigr in salivary glands. This study suggests a new direction for ASD diagnosis and prevention of oral diseases in ASD cohorts and provides evidence for the ASD mucosal immunophenotype in the oral cavity.
Keywords: Streptococcus mutans; autism spectrum disorders; behavior; immunoglobulin A; mucosal immunity; polymeric immunoglobulin receptor; saliva.
Copyright © 2021 Gong, Qiao, Li, Zheng, Xu, Wang, Mi and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Polymeric immunoglobulin receptor expression and local immunoglobulin A production in bovine sublingual, submandibular and parotid salivary glands.Vet J. 2013 Aug;197(2):291-6. doi: 10.1016/j.tvjl.2012.12.030. Epub 2013 Feb 6. Vet J. 2013. PMID: 23395346
-
Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats.Int J Mol Sci. 2020 Jan 27;21(3):815. doi: 10.3390/ijms21030815. Int J Mol Sci. 2020. PMID: 32012687 Free PMC article.
-
Salivary IgA antibody responses to Streptococcus mitis and Streptococcus mutans in preterm and fullterm newborn children.Arch Oral Biol. 2012 Jun;57(6):647-53. doi: 10.1016/j.archoralbio.2011.11.011. Epub 2011 Dec 12. Arch Oral Biol. 2012. PMID: 22169809
-
[Clinical significance of analysis of immunoglobulin A levels in saliva].Med Pregl. 2000 Mar-Apr;53(3-4):164-8. Med Pregl. 2000. PMID: 10965682 Review. Croatian.
-
Do salivary antibodies reliably reflect both mucosal and systemic immunity?Ann N Y Acad Sci. 2007 Mar;1098:288-311. doi: 10.1196/annals.1384.012. Ann N Y Acad Sci. 2007. PMID: 17435136 Review.
Cited by
-
The submandibular and sublingual glands maintain oral microbial homeostasis through multiple antimicrobial proteins.Front Cell Infect Microbiol. 2023 Jan 10;12:1057327. doi: 10.3389/fcimb.2022.1057327. eCollection 2022. Front Cell Infect Microbiol. 2023. PMID: 36704102 Free PMC article.
-
Salivary Immunoglobulin a Alterations in Health and Disease: A Bibliometric Analysis of Diagnostic Trends from 2009 to 2024.Antibodies (Basel). 2024 Nov 29;13(4):98. doi: 10.3390/antib13040098. Antibodies (Basel). 2024. PMID: 39727481 Free PMC article.
-
Exploratory focused pharmacogenetic testing reveals novel markers associated with risperidone pharmacokinetics in Saudi children with autism.Front Pharmacol. 2024 Feb 5;15:1356763. doi: 10.3389/fphar.2024.1356763. eCollection 2024. Front Pharmacol. 2024. PMID: 38375040 Free PMC article.
-
Identifying Rare Genetic Variants of Immune Mediators as Risk Factors for Autism Spectrum Disorder.Genes (Basel). 2022 Jun 20;13(6):1098. doi: 10.3390/genes13061098. Genes (Basel). 2022. PMID: 35741860 Free PMC article.
References
-
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. . Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. (2018) 67:1–23. 10.15585/mmwr.ss6706a1 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

