Proglucagon-Derived Peptides as Therapeutics
- PMID: 34093449
- PMCID: PMC8171296
- DOI: 10.3389/fendo.2021.689678
Proglucagon-Derived Peptides as Therapeutics
Abstract
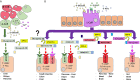
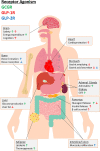
Initially discovered as an impurity in insulin preparations, our understanding of the hyperglycaemic hormone glucagon has evolved markedly over subsequent decades. With description of the precursor proglucagon, we now appreciate that glucagon was just the first proglucagon-derived peptide (PGDP) to be characterised. Other bioactive members of the PGDP family include glucagon-like peptides -1 and -2 (GLP-1 and GLP-2), oxyntomodulin (OXM), glicentin and glicentin-related pancreatic peptide (GRPP), with these being produced via tissue-specific processing of proglucagon by the prohormone convertase (PC) enzymes, PC1/3 and PC2. PGDP peptides exert unique physiological effects that influence metabolism and energy regulation, which has witnessed several of them exploited in the form of long-acting, enzymatically resistant analogues for treatment of various pathologies. As such, intramuscular glucagon is well established in rescue of hypoglycaemia, while GLP-2 analogues are indicated in the management of short bowel syndrome. Furthermore, since approval of the first GLP-1 mimetic for the management of Type 2 diabetes mellitus (T2DM) in 2005, GLP-1 therapeutics have become a mainstay of T2DM management due to multifaceted and sustainable improvements in glycaemia, appetite control and weight loss. More recently, longer-acting PGDP therapeutics have been developed, while newfound benefits on cardioprotection, bone health, renal and liver function and cognition have been uncovered. In the present article, we discuss the physiology of PGDP peptides and their therapeutic applications, with a focus on successful design of analogues including dual and triple PGDP receptor agonists currently in clinical development.
Keywords: GLP-1; GLP-2; diabetes; glucagon; multi-agonist; obesity; oxyntomodulin; proglucagon.
Copyright © 2021 Lafferty, O’Harte, Irwin, Gault and Flatt.
Conflict of interest statement
PF, VG, NI,and FO’H are named on patents filed by Ulster University for the exploitation of incretin-based drugs and other peptide therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

