Effect of Different Nuclear Localization Signals on the Subcellular Localization and Anti-HIV-1 Function of the MxB Protein
- PMID: 34093497
- PMCID: PMC8173038
- DOI: 10.3389/fmicb.2021.675201
Effect of Different Nuclear Localization Signals on the Subcellular Localization and Anti-HIV-1 Function of the MxB Protein
Abstract
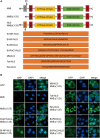
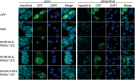
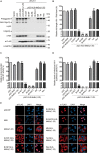
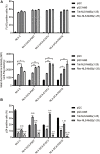
Interferon exerts its antiviral activity by stimulating the expression of antiviral proteins. These interferon stimulate genes (ISGs) often target a group of viruses with unique molecular mechanisms. One such ISG is myxovirus resistance B (MxB) that has been reported to inhibit human immunodeficiency virus type 1 (HIV-1) by targeting viral capsid and impairing nuclear import of viral DNA. The antiviral specificity of MxB is determined by its N-terminal 25 amino acids sequence which has the nuclear localization activity, therefore functions as a nuclear localization signal (NLS). In this study, we report that the bipartite NLS, but not the classic NLS, the PY-NLS, nor the arginine-rich NLS, when used to replace the N-terminal sequence of MxB, drastically suppress HIV-1 gene expression and virus production, thus creates a new anti-HIV-1 mechanism. MxB preserves its anti-HIV-1 activity when its N-terminal sequence is replaced by the arginine-rich NLS. Interestingly, the arginine-rich NLS allows MxB to inhibit HIV-1 CA mutants that are otherwise resistant to wild type MxB, which suggests sequence specific targeting of viral capsid. Together, these data implicate that it is not the nuclear import function itself, but rather the sequence and the mechanism of action of the NLS which define the antiviral property of MxB.
Keywords: HIV-1; MxB; antiviral activity; interferon; nuclear localization signal.
Copyright © 2021 Chai, Wang, Pan, Tan, Qiao and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Host and viral determinants for MxB restriction of HIV-1 infection.Retrovirology. 2014 Oct 25;11:90. doi: 10.1186/s12977-014-0090-z. Retrovirology. 2014. PMID: 25348155 Free PMC article.
-
Subcellular Localization of MxB Determines Its Antiviral Potential against Influenza A Virus.J Virol. 2020 Oct 27;94(22):e00125-20. doi: 10.1128/JVI.00125-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32907985 Free PMC article.
-
Pro-515 of the dynamin-like GTPase MxB contributes to HIV-1 inhibition by regulating MxB oligomerization and binding to HIV-1 capsid.J Biol Chem. 2020 May 8;295(19):6447-6456. doi: 10.1074/jbc.RA119.012439. Epub 2020 Mar 26. J Biol Chem. 2020. PMID: 32217692 Free PMC article.
-
Mx oligomer: a novel capsid pattern sensor?Future Microbiol. 2016 Aug;11:1047-55. doi: 10.2217/fmb-2016-0004. Epub 2016 Aug 5. Future Microbiol. 2016. PMID: 27492442 Review.
-
Factors influencing the nuclear targeting ability of nuclear localization signals.J Drug Target. 2016 Dec;24(10):927-933. doi: 10.1080/1061186X.2016.1184273. Epub 2016 May 24. J Drug Target. 2016. PMID: 27126810 Review.
Cited by
-
The nuclear localization signal of CPSF6 governs post-nuclear import steps of HIV-1 infection.PLoS Pathog. 2025 Jan 17;21(1):e1012354. doi: 10.1371/journal.ppat.1012354. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39823525 Free PMC article.
-
A new Karyopherin-β2 binding PY-NLS epitope of HNRNPH2 linked to neurodevelopmental disorders.Structure. 2023 Aug 3;31(8):924-934.e4. doi: 10.1016/j.str.2023.05.010. Epub 2023 Jun 5. Structure. 2023. PMID: 37279758 Free PMC article.
-
Coronavirus nucleocapsid protein enhances the binding of p-PKCα to RACK1: Implications for inhibition of nucleocytoplasmic trafficking and suppression of the innate immune response.PLoS Pathog. 2024 Nov 27;20(11):e1012097. doi: 10.1371/journal.ppat.1012097. eCollection 2024 Nov. PLoS Pathog. 2024. PMID: 39602452 Free PMC article.
-
VCY mediates inhibition of PFV replication via interaction with the transcription activator Tas.J Virol. 2025 Jul 22;99(7):e0016625. doi: 10.1128/jvi.00166-25. Epub 2025 Jun 3. J Virol. 2025. PMID: 40459262 Free PMC article.
-
Role of Type I Interferons during Mycobacterium tuberculosis and HIV Infections.Biomolecules. 2024 Jul 14;14(7):848. doi: 10.3390/biom14070848. Biomolecules. 2024. PMID: 39062562 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

