Therapeutic Potential of TNFα and IL1β Blockade for CRS/ICANS in CAR-T Therapy via Ameliorating Endothelial Activation
- PMID: 34093519
- PMCID: PMC8170323
- DOI: 10.3389/fimmu.2021.623610
Therapeutic Potential of TNFα and IL1β Blockade for CRS/ICANS in CAR-T Therapy via Ameliorating Endothelial Activation
Abstract
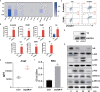
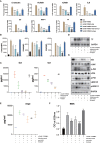
Severe cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) strongly hampered the broad clinical applicability of chimeric antigen receptor T cell (CAR-T) therapy. Vascular endothelial activation has been suggested to contribute to the development of CRS and ICANS after CAR-T therapy. However, therapeutic strategies targeting endothelial dysfunction during CAR-T therapy have not been well studied yet. Here, we found that tumor necrosis factor α (TNFα) produced by CAR-T cells upon tumor recognition and interleukin 1β (IL1β) secreted by activated myeloid cells were the main cytokines in inducing endothelial activation. Therefore, we investigated the potential effectiveness of TNFα and IL1β signaling blockade on endothelial activation in CAR-T therapy. The blockade of TNFα and IL1β with adalimumab and anti-IL1β antibody respectively, as well as the application of focal adhesion kinase (FAK) inhibitor, effectively ameliorated endothelial activation induced by CAR-T, tumor cells, and myeloid cells. Moreover, adalimumab and anti-IL1β antibody exerted synergistic effect on the prevention of endothelial activation induced by CAR-T, tumor cells, and myeloid cells. Our results indicate that TNFα and IL1β blockade might have therapeutic potential for the treatment of CAR-T therapy-associated CRS and neurotoxicity.
Keywords: CAR-T immunotherapy; coagulation; cytokine release; inflammatory response; leakage.
Copyright © 2021 Chen, Li, Shang, Yang, Li, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
The influence of CRS and ICANS on the efficacy of anti-CD19 CAR-T treatment for B-cell acute lymphoblastic leukemia.Front Immunol. 2024 Sep 27;15:1448709. doi: 10.3389/fimmu.2024.1448709. eCollection 2024. Front Immunol. 2024. PMID: 39399502 Free PMC article.
-
Impact of tocilizumab on anti-CD19 chimeric antigen receptor T-cell therapy in B-cell acute lymphoblastic leukemia.Cancer. 2024 Aug 1;130(15):2660-2669. doi: 10.1002/cncr.35316. Epub 2024 Apr 5. Cancer. 2024. PMID: 38578977
-
Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia.J Cell Mol Med. 2021 Dec;25(24):11063-11074. doi: 10.1111/jcmm.17029. Epub 2021 Nov 3. J Cell Mol Med. 2021. PMID: 34734474 Free PMC article.
-
CAR-T Cell Therapy in Cancer: Tribulations and Road Ahead.J Immunol Res. 2020 Jan 17;2020:1924379. doi: 10.1155/2020/1924379. eCollection 2020. J Immunol Res. 2020. PMID: 32411789 Free PMC article. Review.
-
Comprehensive analysis of the efficacy and safety of CAR T-cell therapy in patients with relapsed or refractory B-cell acute lymphoblastic leukaemia: a systematic review and meta-analysis.Ann Med. 2024 Dec;56(1):2349796. doi: 10.1080/07853890.2024.2349796. Epub 2024 May 13. Ann Med. 2024. PMID: 38738799 Free PMC article.
Cited by
-
Selinexor Reduces the Immunosuppression of Macrophages and Synergizes With CD19 CAR-T Cells Against B-Cell Lymphoma.Cancer Sci. 2025 Sep;116(9):2388-2399. doi: 10.1111/cas.70123. Epub 2025 Jun 17. Cancer Sci. 2025. PMID: 40528279 Free PMC article.
-
Bidirectional Communication Between the Innate and Adaptive Immune Systems.Annu Rev Immunol. 2025 Apr;43(1):489-514. doi: 10.1146/annurev-immunol-083122-040624. Annu Rev Immunol. 2025. PMID: 40279312 Free PMC article. Review.
-
Challenges and strategies in relation to effective CAR-T cell immunotherapy for solid tumors.Med Oncol. 2024 Apr 23;41(5):126. doi: 10.1007/s12032-024-02310-y. Med Oncol. 2024. PMID: 38652178 Review.
-
Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies.J Exp Clin Cancer Res. 2021 Nov 18;40(1):367. doi: 10.1186/s13046-021-02148-6. J Exp Clin Cancer Res. 2021. PMID: 34794490 Free PMC article. Review.
-
Biomarkers for prediction of CAR T therapy outcomes: current and future perspectives.Front Immunol. 2024 Mar 15;15:1378944. doi: 10.3389/fimmu.2024.1378944. eCollection 2024. Front Immunol. 2024. PMID: 38558801 Free PMC article. Review.
References
-
- Boyiadzis MM, Dhodapkar MV, Brentjens RJ, Kochenderfer JN, Neelapu SS, Maus MV, et al. . Chimeric Antigen Receptor (CAR) T Therapies for the Treatment of Hematologic Malignancies: Clinical Perspective and Significance. J Immunother Cancer (2018) 6(1):137. 10.1186/s40425-018-0460-5 - DOI - PMC - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. . Lisocabtagene Maraleucel for Patients With Relapsed or Refractory Large B-Cell Lymphomas (TRANSCEND NHL 001): A Multicentre Seamless Design Study. Lancet (2020) 396(10254):839–52. 10.1016/s0140-6736(20)31366-0 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

