Remdesivir Alleviates Acute Kidney Injury by Inhibiting the Activation of NLRP3 Inflammasome
- PMID: 34093539
- PMCID: PMC8176923
- DOI: 10.3389/fimmu.2021.652446
Remdesivir Alleviates Acute Kidney Injury by Inhibiting the Activation of NLRP3 Inflammasome
Abstract
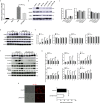
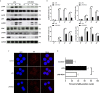
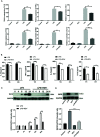
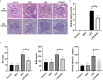
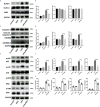
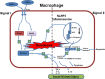
Acute kidney injury (AKI) is a frequent clinical complication in critically ill patients, and it rapidly develops into renal failure with high morbidity and mortality. However, other than dialysis, no effective therapeutic interventions can offer reliable treatment to limit renal injury and improve survival. Here, we firstly reported that remdesivir (RDV, GS-5734), a broad-spectrum antiviral nucleotide prodrug, alleviated AKI by specifically inhibiting NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome activation in macrophages. Mechanically, RDV effectively suppressed the activities of nuclear transcription factor (NF)-κB, mitogen-activated protein kinase (MAPK), which further led to the reduction of the inflammasome genes of NLRP3 transcription, limiting the activation of NLRP3 inflammasome in vivo and in vitro. RDV also inhibited other pro-inflammatory genes including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-12, IL-1β, and interferon-β (IFN-β), leading to the reduction of inflammatory factors release. Thus, RDV can ameliorate AKI via modulating macrophage inflammasome activation and inflammatory immune responses and may have a therapeutic potential for patients with AKI in clinical application.
Keywords: LRR-; NF-κB; NLRP3 inflammasome or NOD-; acute kidney injury; macrophage; pyrin domain-containing protein 3 (NLRP3)inflammasome; remdesivir.
Copyright © 2021 Yin, Zhao, Zhang, Li, Dong, Ju, Kong and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
NLRP3 inflammasome activation regulated by NF-κB and DAPK contributed to paraquat-induced acute kidney injury.Immunol Res. 2017 Jun;65(3):687-698. doi: 10.1007/s12026-017-8901-7. Immunol Res. 2017. PMID: 28215032
-
MIF inhibitor ISO-1 alleviates severe acute pancreatitis-associated acute kidney injury by suppressing the NLRP3 inflammasome signaling pathway.Int Immunopharmacol. 2021 Jul;96:107555. doi: 10.1016/j.intimp.2021.107555. Epub 2021 Apr 3. Int Immunopharmacol. 2021. PMID: 33823428
-
Dopamine D1 receptor agonist A68930 attenuates acute kidney injury by inhibiting NLRP3 inflammasome activation.J Pharmacol Sci. 2020 Jul;143(3):226-233. doi: 10.1016/j.jphs.2020.04.005. Epub 2020 Apr 18. J Pharmacol Sci. 2020. PMID: 32446726
-
Role and mechanism of endoplasmic reticulum stress and NLRP3 inflammasome in acute kidney injury.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Mar 28;49(3):367-376. doi: 10.11817/j.issn.1672-7347.2024.230301. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38970510 Free PMC article. Review. Chinese, English.
-
The roles of NLRP3 inflammasome-mediated signaling pathways in hyperuricemic nephropathy.Mol Cell Biochem. 2021 Mar;476(3):1377-1386. doi: 10.1007/s11010-020-03997-z. Epub 2021 Jan 3. Mol Cell Biochem. 2021. PMID: 33389490 Review.
Cited by
-
Inflammasome pathway in kidney transplantation.Front Med (Lausanne). 2023 Nov 8;10:1303110. doi: 10.3389/fmed.2023.1303110. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38020086 Free PMC article. Review.
-
Remdesivir in the Treatment of COVID-19: A Propensity Score-Matched Analysis from a Public Hospital in New York City Assessing Renal and Hepatic Safety.J Clin Med. 2022 May 31;11(11):3132. doi: 10.3390/jcm11113132. J Clin Med. 2022. PMID: 35683518 Free PMC article.
-
Monitoring Macrophage Polarization in Infectious Disease, Lesson From SARS-CoV-2 Infection.Rev Med Virol. 2025 May;35(3):e70034. doi: 10.1002/ird3.70006. Rev Med Virol. 2025. PMID: 40148134 Free PMC article. Review.
-
Remdesivir inhibits endothelial activation and atherosclerosis by coupling TAL1 to TRAF6.J Transl Med. 2025 Jul 1;23(1):719. doi: 10.1186/s12967-025-06673-2. J Transl Med. 2025. PMID: 40598260 Free PMC article.
-
Molecular mechanisms and therapeutic interventions in acute kidney injury: a literature review.BMC Nephrol. 2025 Mar 22;26(1):144. doi: 10.1186/s12882-025-04077-4. BMC Nephrol. 2025. PMID: 40121405 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

