Effects of Peptide-Induced Immune Tolerance on Murine Lupus
- PMID: 34093553
- PMCID: PMC8171184
- DOI: 10.3389/fimmu.2021.662901
Effects of Peptide-Induced Immune Tolerance on Murine Lupus
Abstract
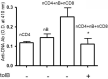
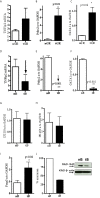
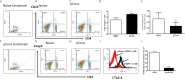
The regulation of autoimmunity and the molecular mechanisms by which different immune cells, including T cells, polymorphonuclear leukocytes (PMN-granulocytes), and B cells suppress autoimmune diseases is complex. We have shown previously that BWF1 lupus mice are protected from autoimmunity after i.v. injection or oral administration of tolerogenic doses of pCons, an artificial synthetic peptide based on sequences containing MHC class I and MHC class II determinants in the VH region of a J558-encoded BWF1 anti-DNA Ab. Several T cell subsets can transfer this tolerance. In this study, we determined the potential roles of granulocytes, B cells and regulatory T cells altered by pCons treatment in the BWF1 (NZB/NZW) mouse model of lupus. Immunophenotyping studies indicated that pCons treatment of BWF1 mice significantly increased CD4+FoxP3+ T cells, reduced the percent of B cells expressing CD19+CD5+ but increased the percent of CD19+CD1d+ regulatory B cells and increased the ability of the whole B cell population to suppress IgG anti-DNA production in vitro. pCons treatment significantly decreased the expression of CTLA-4 (cytotoxic T-lymphocyte-associated protein-4) in CD8+ T cells. In addition, peptide administration modified granulocytes so they became suppressive. We co-cultured sorted naïve B cells from mice making anti-DNA Ab (supported by addition of sorted naive CD4+ and CD8+ T cells from young auto-antibody-negative BWF1 mice) with sorted B cells or granulocytes from tolerized mice. Both tolerized granulocytes and tolerized B cells significantly suppressed the production of anti-DNA in vitro. In granulocytes from tolerized mice compared to saline-treated littermate controls, real-time PCR analysis indicated that expression of interferon-induced TNFAIP2 increased more than 2-fold while Ptdss2 and GATA1 mRNA were up-regulated more than 10-fold. In contrast, expression of these genes was significantly down-regulated in tolerized B cells. Further, another IFN-induced protein, Bcl2, was reduced in tolerized B cells as determined by Western blot analyses. In contrast, expression of FoxP3 was significantly increased in tolerized B cells. Together, these data suggest that B cells and granulocytes are altered toward suppressive functions by in vivo tolerization of BWF1 mice with pCons and it is possible these cell types participate in the clinical benefits seen in vivo.
Keywords: Anti-DNA Ab; granulocytes; immune tolerance and regulation; pConsensus peptide (pCons); polymorphonuclear cells (PMNs); regulatory B cells; systemic lupus erythematosus.
Copyright © 2021 Singh, Hahn and Bischoff.
Conflict of interest statement
BHH and RPS have a patent through the University of California, Los Angeles for the use of pCons as an immune modulator in systemic lupus erythematosus. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

