Management of Donor-Specific Antibodies in Haploidentical Transplant: Multicenter Experience From the Madrid Group of Hematopoietic Transplant
- PMID: 34093576
- PMCID: PMC8170127
- DOI: 10.3389/fimmu.2021.674658
Management of Donor-Specific Antibodies in Haploidentical Transplant: Multicenter Experience From the Madrid Group of Hematopoietic Transplant
Abstract
Background: Donor specific antibodies (DSAs) can be responsible for graft failure (GF) in the setting of mismatched hematopoietic stem cell transplantation (HSCT). The aim of our study is to report the experience of the Madrid Group of Hematopoietic Transplant (GMTH) in patients with DSAs undergoing haplo-HSCT.
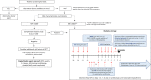
Methods: Patients undergoing haplo-HSCT in centers from the GMTH from 2012 to 2020 were included in the study. DSAs were analyzed with a solid-phase single-antigen immunoassay; monitoring was performed during desensitization on days -14, -7, 0 and in a weekly basis until neutrophil engraftment. Desensitization strategies varied depending on center experience, immunofluorescence intensity, complement fixation and type of antibodies.
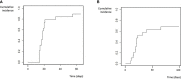
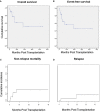
Results: We identified a total of 20 haplo-HSCT in 19 patients performed with DSAs in 5 centers. 10 (53%) patients presented anti-HLA class I DSAs (6 of them with > 5000 mean fluorescence intensity (MFI)), 4 (21%) presented anti-HLA class II (1 with > 5000 MFI) and 5 (26%) presented both anti-HLA class I and II (5 with > 5000 MFI). 90% of patients received at least two treatments as desensitization strategy and all experienced a decrease of MFI after desensitization (mean reduction 74%). Only one patient who developed progressive increase of MFI after infusion developed GF. Desensitization treatments used included rituximab, immunoglobulins, therapeutic plasma exchange, incompatible platelets, buffy coat and immunosuppressors. Seventeen (90%) patients achieved neutrophil engraftment; one patient died before engraftment because of infection and one patient with class I DSAs developed primary GF despite an intensive desensitization. After a median follow-up of 10 months, OS and EFS were 60% and 58%, respectively, cumulative incidence of relapse was 5% and NRM was 32%.
Conclusions: Despite the optimal strategy of DSAs desensitization remains unclear, the use of desensitization treatment guided by DSAs intensity kinetics constitute an effective approach with high rates of engraftment for patients with DSAs in need for an haplo-HSCT lacking an alternative suitable donor.
Keywords: Luminex ®; desensitization therapy; donor-specific anti HLA antibodies; haplo identical hematopoietic stem cell transplantation; kinetics.
Copyright © 2021 Bailén, Vicario, Solán, Sánchez-Vadillo, Herrera, Calbacho, Alenda, López-Lorenzo, Humala, Chinea, Sánchez-Pina, Balas, Moreno, Arzuaga, Pradillo, Dorado, Oarbeascoa, Anguita, Díez-Martín and Kwon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Center for International Blood and Marrow Transplant Research (CIBMTR) Annual Report . Cibmtr Annual Report. (2019). Available at: https://www.cibmtr.org/About/AdminReports/Pages/index.aspx
-
- Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The Detection of Donor-Directed, HLA-Specific Alloantibodies in Recipients of Unrelated Hematopoietic Cell Transplantation is Predictive of Graft Failure. Blood (2010) 115:5. 10.1182/blood-2009-09-244525 - DOI - PMC - PubMed
-
- Luznik L, Jalla S, Engstrom L, Iannone R, Fuchs EJ. Durable Engraftment of Major Histocompatibility Complex-Incompatible Cells After Nonmyeloablative Conditioning With Fludarabine, Low-Dose Total Body Irradiation, and Posttransplantation Cyclophosphamide. Blood (2001) 98:3456–64. 10.1182/blood.V98.12.3456 - DOI - PubMed
-
- Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. Hla-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol Blood Marrow Transplant (2008) 14:641–50. 10.1016/j.bbmt.2008.03.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

