CD47 Deficiency Ameliorates Ocular Autoimmune Inflammation
- PMID: 34093583
- PMCID: PMC8174453
- DOI: 10.3389/fimmu.2021.680568
CD47 Deficiency Ameliorates Ocular Autoimmune Inflammation
Abstract
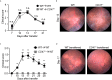
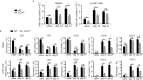
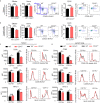
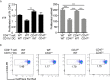
Autoimmune uveitis is a sight-threatening ocular inflammatory condition in which the retina and uveal tissues become a target of autoreactive immune cells. The CD47 is a ubiquitously expressed transmembrane protein which plays multiple roles in fundamental cellular functions including phagocytosis, proliferation, and adhesion. Signal regulatory protein alpha (SIRPα), one of the CD47 ligands, is predominantly expressed in myeloid lineage cells such as dendritic cells (DCs) or macrophages, and CD47-SIRPα signaling pathway is implicated in the development of autoimmune diseases. Our current study demonstrates how CD47 depletion is effective in the prevention of experimental autoimmune uveitis (EAU), an animal model of human autoimmune uveitis, in animals deficient of CD47 (CD47-/- ). Systemic suppression of SIRPα+ DCs in animals deficient in CD47 resulted in the inability of autoreactive CD4+ T cells to develop, which is crucial to induction of EAU. Of interest, retinal microglia, the resident immune cell of the retina, express SIRPα, however these cells were not operative in EAU suppression in response to CD47 depletion. These results identify CD47 as a significant regulator in the development of SIRPα+ DCs that is vital to disease induction in EAU.
Keywords: CD47 antigen; antigen-presenting cells; autoimmune diseases; retina; uveitis.
Copyright © 2021 Okunuki, Tabor, Lee and Connor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

