IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment
- PMID: 34093586
- PMCID: PMC8176033
- DOI: 10.3389/fimmu.2021.683332
IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment
Abstract
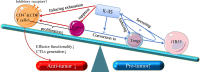
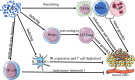
Interleukin-35 (IL-35) is a heterodimeric cytokine composed of Epstein-Barr virus-induced gene 3 (EBI3) and IL-12p35 that has recently been shown to play diverse and important roles in the tumor microenvironment (TME). Owing to its immunosuppressive activity and ability to promote tumor growth and progression, IL-35 is widely recognized as a key mediator of TME status. Immune cells are key mediators of diverse tumor-related phenotypes, and immunosuppressive cytokines such as IL-35 can promote tumor growth and metastasis in TME. These influences should be considered together. Since tumor immunotherapy based on immune checkpoint blockade remains ineffective in many patients due to tumoral resistance, a new target or efficacy enhancing factor is urgently needed. Suppressing IL-35 production and activity has been demonstrated as an effective factor that inhibits tumor cells viability, and further investigation of this cytokine is warranted. However, the mechanistic basis for IL-35-mediated regulation of immune cells in the TME remains to be fully clarified. In the present review, we explore the roles of IL-35 in regulating immune cells within the TME. In addition, we highlight IL-35 as a specific immunological target and discuss its possible relevance in the context of immunotherapy. Lastly, we sought to summarize potential future research directions that may guide the advancement of current understanding regarding the role of this important cytokine as a regulator of oncogenesis.
Keywords: IL-35; anti-tumor immunity; regulatory immune cells; tumor immunotherapy; tumor microenvironment.
Copyright © 2021 Liu, Huang, Nie, Tan, Xing, Qu and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives.Mol Cancer. 2021 Oct 11;20(1):131. doi: 10.1186/s12943-021-01428-1. Mol Cancer. 2021. PMID: 34635121 Free PMC article. Review.
-
The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment.Front Immunol. 2021 Apr 27;12:656908. doi: 10.3389/fimmu.2021.656908. eCollection 2021. Front Immunol. 2021. PMID: 33986746 Free PMC article. Review.
-
Hijacked Immune Cells in the Tumor Microenvironment: Molecular Mechanisms of Immunosuppression and Cues to Improve T Cell-Based Immunotherapy of Solid Tumors.Int J Mol Sci. 2021 May 27;22(11):5736. doi: 10.3390/ijms22115736. Int J Mol Sci. 2021. PMID: 34072260 Free PMC article. Review.
-
The Cancer Cell Dissemination Machinery as an Immunosuppressive Niche: A New Obstacle Towards the Era of Cancer Immunotherapy.Front Immunol. 2021 Apr 13;12:654877. doi: 10.3389/fimmu.2021.654877. eCollection 2021. Front Immunol. 2021. PMID: 33927723 Free PMC article. Review.
-
The circuitry of the tumor microenvironment in adult and pediatric Hodgkin lymphoma: cellular composition, cytokine profile, EBV, and exosomes.Cancer Rep (Hoboken). 2021 Apr;4(2):e1311. doi: 10.1002/cnr2.1311. Epub 2020 Oct 26. Cancer Rep (Hoboken). 2021. PMID: 33103852 Free PMC article. Review.
Cited by
-
Regulatory T lymphocytes as a therapy for ischemic stroke.Semin Immunopathol. 2023 May;45(3):329-346. doi: 10.1007/s00281-022-00975-z. Epub 2022 Dec 5. Semin Immunopathol. 2023. PMID: 36469056 Free PMC article. Review.
-
Importance of the Immune Microenvironment in the Spontaneous Regression of Cervical Squamous Intraepithelial Lesions (cSIL) and Implications for Immunotherapy.J Clin Med. 2022 Mar 5;11(5):1432. doi: 10.3390/jcm11051432. J Clin Med. 2022. PMID: 35268523 Free PMC article. Review.
-
Role of IL-27 in COVID-19: A Thin Line between Protection and Disease Promotion.Int J Mol Sci. 2024 Jul 21;25(14):7953. doi: 10.3390/ijms25147953. Int J Mol Sci. 2024. PMID: 39063193 Free PMC article. Review.
-
Focusing on scRNA-seq-Derived T Cell-Associated Genes to Identify Prognostic Signature and Immune Microenvironment Status in Low-Grade Glioma.Mediators Inflamm. 2023 May 31;2023:3648946. doi: 10.1155/2023/3648946. eCollection 2023. Mediators Inflamm. 2023. PMID: 37292257 Free PMC article.
-
Gut microbiota CLA and IL-35 induction in macrophages through Gαq/11-mediated STAT1/4 pathway: an animal-based study.Gut Microbes. 2024 Jan-Dec;16(1):2437253. doi: 10.1080/19490976.2024.2437253. Epub 2024 Dec 5. Gut Microbes. 2024. PMID: 39636005 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

