Impaired Vitamin D Signaling in T Cells From a Family With Hereditary Vitamin D Resistant Rickets
- PMID: 34093587
- PMCID: PMC8170129
- DOI: 10.3389/fimmu.2021.684015
Impaired Vitamin D Signaling in T Cells From a Family With Hereditary Vitamin D Resistant Rickets
Abstract
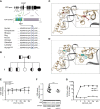
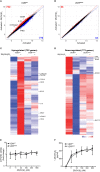
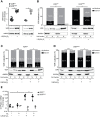
The active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), mediates its immunomodulatory effects by binding to the vitamin D receptor (VDR). Here, we describe a new point mutation in the DNA-binding domain of the VDR and its consequences for 1,25(OH)2D3 signaling in T cells from heterozygous and homozygous carriers of the mutation. The mutation did not affect the overall structure or the ability of the VDR to bind 1,25(OH)2D3 and the retinoid X receptor. However, the subcellular localization of the VDR was strongly affected and the transcriptional activity was abolished by the mutation. In heterozygous carriers of the mutation, 1,25(OH)2D3-induced gene regulation was reduced by ~ 50% indicating that the expression level of wild-type VDR determines 1,25(OH)2D3 responsiveness in T cells. We show that vitamin D-mediated suppression of vitamin A-induced gene regulation depends on an intact ability of the VDR to bind DNA. Furthermore, we demonstrate that vitamin A inhibits 1,25(OH)2D3-induced translocation of the VDR to the nucleus and 1,25(OH)2D3-induced up-regulation of CYP24A1. Taken together, this study unravels novel aspects of vitamin D signaling and function of the VDR in human T cells.
Keywords: HVDRR; T cells; vitamin A; vitamin D; vitamin D receptor.
Copyright © 2021 Al-Jaberi, Kongsbak-Wismann, Aguayo-Orozco, Krogh, Buus, Lopez, Rode, Gravesen, Olgaard, Brunak, Woetmann, Ødum, Bonefeld and Geisler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Rode AKO, Kongsbak M, Hansen MM, Lopez DV, Levring TB, Woetmann A, et al. Vitamin D Counteracts Mycobacterium Tuberculosis-Induced Cathelicidin Downregulation in Dendritic Cells and Allows Th1 Differentiation and IFNgamma Secretion. Front Immunol (2017) 8:656. 10.3389/fimmu.2017.00656 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

