12/15-Lipoxygenase Regulates IL-33-Induced Eosinophilic Airway Inflammation in Mice
- PMID: 34093589
- PMCID: PMC8170304
- DOI: 10.3389/fimmu.2021.687192
12/15-Lipoxygenase Regulates IL-33-Induced Eosinophilic Airway Inflammation in Mice
Abstract
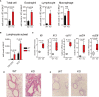
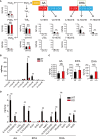
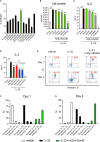
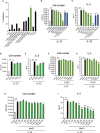
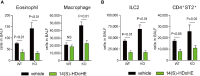
Dysregulated fatty acid metabolism is clinically associated with eosinophilic allergic diseases, including severe asthma and chronic rhinosinusitis. This study aimed to demonstrate the role of 12/15-lipoxygenase (12/15-LOX) in interleukin (IL)-33-induced eosinophilic airway inflammation; to this end, we used 12/15-LOX-deficient mice, which displayed augmented IL-33-induced lung inflammation, characterized by an increased number of infiltrated eosinophils and group 2 innate lymphoid cells (ILC2s) in the airway. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based lipidomics revealed that the levels of a series of 12/15-LOX-derived metabolites were significantly decreased, and application of 14(S)-hydroxy docosahexaenoic acid (HDoHE), a major 12/15-LOX-derived product, suppressed IL-33-mediated eosinophilic inflammation in 12/15-LOX-deficient mice. Using bioactive lipid screening, we found that 14(S)-HDoHE and 10(S),17(S)-diHDoHE markedly attenuated ILC2 proliferation and cytokine production at micromolar concentration in vitro. In addition, maresin 1 (MaR1) and resolvin D1 (RvD1), 12/15-LOX-derived specialized proresolving mediators (SPMs), inhibited cytokine production of ILC2s at nanomolar concentration. These findings demonstrate the protective role of endogenous 12/15-LOX-derived lipid mediators in controlling ILC2-mediated eosinophilic airway inflammation and related diseases. Thus, 12/15-LOX-derived lipid mediators may represent a potential therapeutic strategy for ameliorating airway inflammation-associated conditions.
Keywords: 12/15-lipoxygenase; 14(S)-HDoHE; IL-33; docosahexaenoic acid; group 2 innate lymphoid cell; lipidomics; maresin; specialized proresolving mediator.
Copyright © 2021 Miyata, Yokokura, Moro, Arai, Fukunaga and Arita.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

