PARK7 Protects Against Chronic Kidney Injury and Renal Fibrosis by Inducing SOD2 to Reduce Oxidative Stress
- PMID: 34093596
- PMCID: PMC8176114
- DOI: 10.3389/fimmu.2021.690697
PARK7 Protects Against Chronic Kidney Injury and Renal Fibrosis by Inducing SOD2 to Reduce Oxidative Stress
Abstract
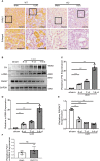
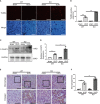
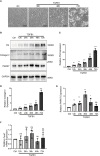
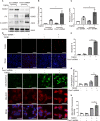
Renal fibrosis is the final common pathway to chronic kidney diseases regardless of etiology. Parkinson disease protein 7 (PARK7) is a multifunctional protein involved in various cellular processes, but its pathophysiological role in kidneys remain largely unknown. Here, we have determined the role of PARK7 in renal fibrosis and have further elucidated the underlying mechanisms by using the in vivo mouse model of unilateral ureteric obstruction (UUO) and the in vitro model of transforming growth factor-b (TGFB1) treatment of cultured kidney proximal tubular cells. PARK7 decreased markedly in atrophic kidney tubules in UUO mice, and Park7 deficiency aggravated UUO-induced renal fibrosis, tubular cell apoptosis, ROS production and inflammation. In vitro, TGFB1 treatment induced fibrotic changes in renal tubular cells, which was accompanied by alterations of PARK7. Park7 knockdown exacerbated TGFB1-induced fibrotic changes, cell apoptosis and ROS production, whereas Park7 overexpression or treatment with ND-13 (a PARK7-derived peptide) attenuated these TGFB1-induced changes. Mechanistically, PARK7 translocated into the nucleus of renal tubular cells following TGFB1 treatment or UUO, where it induced the expression of SOD2, an antioxidant enzyme. Taken together, these results indicate that PARK7 protects against chronic kidney injury and renal fibrosis by inducing SOD2 to reduce oxidative stress in tubular cells.
Keywords: PARK7; antioxidant; reactive oxygen species; renal fibrosis; tubular cells.
Copyright © 2021 Yin, Li, Liu, Wu, Cai, Tang and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

