Phytosterol Profiles, Genomes and Enzymes - An Overview
- PMID: 34093623
- PMCID: PMC8172173
- DOI: 10.3389/fpls.2021.665206
Phytosterol Profiles, Genomes and Enzymes - An Overview
Abstract
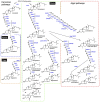
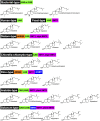
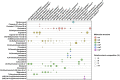
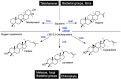
The remarkable diversity of sterol biosynthetic capacities described in living organisms is enriched at a fast pace by a growing number of sequenced genomes. Whereas analytical chemistry has produced a wealth of sterol profiles of species in diverse taxonomic groups including seed and non-seed plants, algae, phytoplanktonic species and other unicellular eukaryotes, functional assays and validation of candidate genes unveils new enzymes and new pathways besides canonical biosynthetic schemes. An overview of the current landscape of sterol pathways in the tree of life is tentatively assembled in a series of sterolotypes that encompass major groups and provides also peculiar features of sterol profiles in bacteria, fungi, plants, and algae.
Keywords: algae; cholesterol; eukaryote; phytosterol; plant; prokaryote.
Copyright © 2021 Darnet, Blary, Chevalier and Schaller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Sterol Biosynthesis in Four Green Algae: A Bioinformatic Analysis of the Ergosterol Versus Phytosterol Decision Point.J Phycol. 2021 Aug;57(4):1199-1211. doi: 10.1111/jpy.13164. Epub 2021 May 20. J Phycol. 2021. PMID: 33713347 Free PMC article.
-
Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy-3-methylglutaryl-CoA reductase.Plant Biotechnol J. 2003 Mar;1(2):113-21. doi: 10.1046/j.1467-7652.2003.00011.x. Plant Biotechnol J. 2003. PMID: 17147748
-
Phylogenomics of sterol synthesis: insights into the origin, evolution, and diversity of a key eukaryotic feature.Genome Biol Evol. 2009 Sep 10;1:364-81. doi: 10.1093/gbe/evp036. Genome Biol Evol. 2009. PMID: 20333205 Free PMC article.
-
Dissecting cholesterol and phytosterol biosynthesis via mutants and inhibitors.J Exp Bot. 2021 Feb 2;72(2):241-253. doi: 10.1093/jxb/eraa429. J Exp Bot. 2021. PMID: 32929492 Review.
-
Sterol methyl transferase: enzymology and inhibition.Biochim Biophys Acta. 2000 Dec 15;1529(1-3):63-88. doi: 10.1016/s1388-1981(00)00138-4. Biochim Biophys Acta. 2000. PMID: 11111078 Review.
Cited by
-
Host cholesterol influences the activity of sterol biosynthesis inhibitors in Leishmania amazonensis.Mem Inst Oswaldo Cruz. 2022 Apr 4;117:e220407. doi: 10.1590/0074-02760220407. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35384972 Free PMC article.
-
Evolutionary formation of melatonin and vitamin D in early life forms: insects take centre stage.Biol Rev Camb Philos Soc. 2024 Oct;99(5):1772-1790. doi: 10.1111/brv.13091. Epub 2024 Apr 30. Biol Rev Camb Philos Soc. 2024. PMID: 38686544 Review.
-
Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells.Biomolecules. 2024 Apr 30;14(5):542. doi: 10.3390/biom14050542. Biomolecules. 2024. PMID: 38785949 Free PMC article.
-
Steroidal Antimetabolites Protect Mice against Trypanosoma brucei.Molecules. 2022 Jun 25;27(13):4088. doi: 10.3390/molecules27134088. Molecules. 2022. PMID: 35807334 Free PMC article.
-
Advances and Challenges in Plant Sterol Research: Fundamentals, Analysis, Applications and Production.Molecules. 2023 Sep 8;28(18):6526. doi: 10.3390/molecules28186526. Molecules. 2023. PMID: 37764302 Free PMC article. Review.
References
-
- Akihisa T., Hayashi Y., Patterson G. W., Shimizu N., Tamura T. (1992). 4α-methylvernosterol and other sterols from Vernonia anthelmintica seeds. Phytochemistry 31 1759–1763.
-
- Alam M. (1979). Dinoflagellate sterols I: sterol composition of the dinoflagellates of ? species. Steroids 33 197–203. - PubMed
-
- Amo M., Suzuki N., Kawamura H., Yamaguchi A., Takano Y., Horiguchi T. (2010). Sterol composition of dinoflagellates: different abundance and composition in heterotrophic species and resting cysts. Geochem. J. 44 225–231.
-
- Babiychuk E., Bouvier-Nave P., Compagnon V., Suzuki M., Muranaka T., Van Montagu M., et al. (2008). Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc. Natl. Acad. Sci. U.S.A. 105 3163–3168. 10.1073/pnas.0712190105 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

