High SEC61G expression predicts poor prognosis in patients with Head and Neck Squamous Cell Carcinomas
- PMID: 34093796
- PMCID: PMC8176234
- DOI: 10.7150/jca.51467
High SEC61G expression predicts poor prognosis in patients with Head and Neck Squamous Cell Carcinomas
Abstract
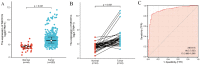
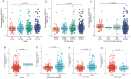
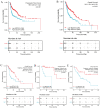
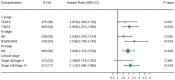
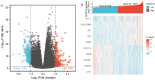
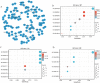
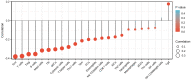
Background: Overexpression of the membrane protein SEC61 translocon gamma subunit (SEC61G) has been observed in a variety of cancers; however, its role in head and neck squamous cell carcinomas (HNSCC) is unknown. This study aimed to elucidate the relationship between SEC61G and HNSCC based on data from The Cancer Genome Atlas (TCGA) database. Methods: Data for HNSCC patients were collected from TCGA and the expression level of SEC61G was compared between paired HNSCC and normal tissues using the Wilcoxon rank-sum test. The relationship between clinicopathologic features and SEC61G expression was also analyzed using the Wilcoxon rank-sum test and logistic regression. Receiver operating characteristic (ROC) curves were generated to evaluate the value of SEC61G as a binary classifier using the area under the curve (AUC value). The association of clinicopathologic characteristics with prognosis in HNSCC patients was assessed using Cox regression and the Kaplan-Meier methods. A nomogram, based on Cox multivariate analysis, was used to predict the impact of SEC61G on prognosis. Functional enrichment analysis was performed to determine the hallmark pathways associated with differentially expressed genes in HNSCC patients exhibiting high and low SEC61G expression. Results: The expression of SEC61G was significantly elevated in HNSCC tissues compared to normal tissues (P < 0.001). The high expression of SEC61G was significantly correlated with the T stage, M stage, clinical stage, TP53 mutation status, PIK3CA mutation status, primary therapy outcome, and cervical lymph node dissection (all P < 0.05). Meanwhile, ROC curves suggested the significant diagnostic ability of SEC61G for HNSCC (AUC = 0.923). Kaplan-Meier survival analysis showed that patients with HNSCC characterized by high SEC61G expression had a poorer prognosis than patients with low SEC61G expression (hazard ratio = 1.95, 95% confidence interval 1.48-2.56, P < 0.001). Univariate and multivariate analyses revealed that SEC61G was independently associated with overall survival (P = 0.027). Functional annotations indicated that SEC61G is involved in pathways related to translation and regulation of SLITs/ROBOs expression, SRP-dependent co-translational protein targeting to the membrane, nonsense-mediated decay, oxidative phosphorylation, and Parkinson's disease. Conclusion: SEC61G plays a vital role in HNSCC progression and prognosis; it may, therefore, serve as an effective biomarker for the prediction of patient survival.
Keywords: SEC61G; biomarker; head and neck squamous cell carcinoma; prognosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

