Tanshinone I, a new EZH2 inhibitor restricts normal and malignant hematopoiesis through upregulation of MMP9 and ABCG2
- PMID: 34093860
- PMCID: PMC8171091
- DOI: 10.7150/thno.53170
Tanshinone I, a new EZH2 inhibitor restricts normal and malignant hematopoiesis through upregulation of MMP9 and ABCG2
Abstract
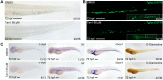
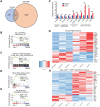
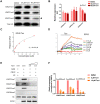
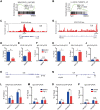
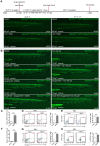
Rationale: Tanshinone, a type of diterpenes derived from salvia miltiorrhiza, is a particularly promising herbal medicine compound for the treatment of cancers including acute myeloid leukemia (AML). However, the therapeutic function and the underlying mechanism of Tanshinone in AML are not clear, and the toxic effect of Tanshinone limits its clinical application. Methods: Our work utilizes human leukemia cell lines, zebrafish transgenics and xenograft models to study the cellular and molecular mechanisms of how Tanshinone affects normal and abnormal hematopoiesis. WISH, Sudan Black and O-Dianisidine Staining were used to determine the expression of hematopoietic genes on zebrafish embryos. RNA-seq analysis showed that differential expression genes and enrichment gene signature with Tan I treatment. The surface plasmon resonance (SPR) method was used with a BIAcore T200 (GE Healthcare) to measure the binding affinities of Tan I. In vitro methyltransferase assay was performed to verify Tan I inhibits the histone enzymatic activity of the PRC2 complex. ChIP-qPCR assay was used to determine the H3K27me3 level of EZH2 target genes. Results: We found that Tanshinone I (Tan I), one of the Tanshinones, can inhibit the proliferation of human leukemia cells in vitro and in the xenograft zebrafish model, as well as the normal and malignant definitive hematopoiesis in zebrafish. Mechanistic studies illustrate that Tan I regulates normal and malignant hematopoiesis through direct binding to EZH2, a well-known histone H3K27 methyltransferase, and inhibiting PRC2 enzymatic activity. Furthermore, we identified MMP9 and ABCG2 as two possible downstream genes of Tan I's effects on EZH2. Conclusions: Together, this study confirmed that Tan I is a novel EZH2 inhibitor and suggested MMP9 and ABCG2 as two potential therapeutic targets for myeloid malignant diseases.
Keywords: EZH2; H3K27me3; Tanshinone I; hematopoiesis; leukemia.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. - PubMed
-
- Cao R, Wang L, Wang H, Xia L, Hediye EB, Paul T. et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298(5595):1039–43. - PubMed
-
- Mochizuki-Kashio M, Mishima Y, Miyagi S, Negishi M, Saraya A, Konuma T. et al. Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood. 2011;118(25):6553–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

